The protein kinase Cdelta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint
- PMID: 19917613
- PMCID: PMC2804346
- DOI: 10.1074/jbc.M109.055392
The protein kinase Cdelta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint
Abstract
Protein kinase Cdelta (PKCdelta) is an essential component of the intrinsic apoptotic program. Following DNA damage, such as exposure to UV radiation, PKCdelta is cleaved in a caspase-dependent manner, generating a constitutively active catalytic fragment (PKCdelta-cat), which is necessary and sufficient for keratinocyte apoptosis. We found that in addition to inducing apoptosis, expression of PKCdelta-cat caused a pronounced G(2)/M cell cycle arrest in both primary human keratinocytes and immortalized HaCaT cells. Consistent with a G(2)/M arrest, PKCdelta-cat induced phosphorylation of Cdk1 (Tyr(15)), a critical event in the G(2)/M checkpoint. Treatment with the ATM/ATR inhibitor caffeine was unable to prevent PKCdelta-cat-induced G(2)/M arrest, suggesting that PKCdelta-cat is functioning downstream of ATM/ATR in the G(2)/M checkpoint. To better understand the role of PKCdelta and PKCdelta-cat in the cell cycle response to DNA damage, we exposed wild-type and PKCdelta null mouse embryonic fibroblasts (MEFs) to UV radiation. Wild-type MEFs underwent a pronounced G(2)/M arrest, Cdk1 phosphorylation, and induction of apoptosis following UV exposure, whereas PKCdelta null MEFs were resistant to these effects. Expression of PKCdelta-green fluorescent protein, but not caspase-resistant or kinase-inactive PKCdelta, was able to restore G(2)/M checkpoint integrity in PKCdelta null MEFs. The function of PKCdelta in the DNA damage-induced G(2)/M cell cycle checkpoint may be a critical component of its tumor suppressor function.
Figures

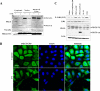
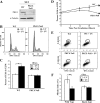
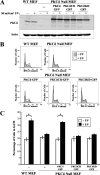

References
-
- Bartek J., Lukas J. (2007) Curr. Opin. Cell Biol. 19, 238–245 - PubMed
-
- Musacchio A., Salmon E. D. (2007) Nat. Rev. Mol. Cell Biol. 8, 379–393 - PubMed
-
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. (1991) Science 253, 49–53 - PubMed
-
- Banin S., Moyal L., Shieh S., Taya Y., Anderson C. W., Chessa L., Smorodinsky N. I., Prives C., Reiss Y., Shiloh Y., Ziv Y. (1998) Science 281, 1674–1677 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

