Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN-{gamma}/JAK/STAT1 signaling is critical for the expression of PTN in macrophages
- PMID: 19917672
- PMCID: PMC2830133
- DOI: 10.1096/fj.09-140780
Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN-{gamma}/JAK/STAT1 signaling is critical for the expression of PTN in macrophages
Abstract
Neovascularization is critical to destabilization of atheroma. We previously reported that the angiogenic growth factor pleiotrophin (PTN) coaxes monocytes to assume the phenotype of functional endothelial cells in vitro and in vivo. In this study we show that PTN expression is colocalized with capillaries of human atherosclerotic plaques. Among the various reagents that are critical to the pathogenesis of atherosclerosis, interferon (IFN)-gamma was found to markedly induce PTN mRNA expression in a dose-dependent manner in macrophages. Mechanistic studies revealed that the Janus kinase inhibitors, WHI-P154 and ATA, efficiently blocked STAT1 phosphorylation in a concentration- and time-dependent manner. Notably, the level of phosphorylated STAT1 was found to correlate directly with the PTN mRNA levels. In addition, STAT1/STAT3/p44/42 signaling molecules were found to be phosphorylated by IFN-gamma in macrophages, and they were translocated into the nucleus. Further, PTN promoter analysis showed that a gamma-activated sequence (GAS) located at -2086 to -2078 bp is essential for IFN-gamma-regulated promoter activity. Moreover, electrophoretic mobility shift, supershift, and chromatin immunoprecipitation analyses revealed that both STAT1 and STAT3 bind to the GAS at the chromatin level in the IFN-gamma stimulated cells. Finally, to test whether the combined effect of STAT1/STAT3/p44/42 signaling is required for the expression of PTN in macrophages, gene knockdowns of these transcription factors were performed using siRNA. Cells lacking STAT1, but not STAT3 or p42, have markedly reduced PTN mRNA levels. These data suggest that PTN expression in the human plaques may be in part regulated by IFN-gamma and that PTN is involved in the adaptive immunity.-Li, F., Tian, F., Wang, L., Williamson, I. K., Sharifi, B. G., Shah, P. K. Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN-gamma/JAK/STAT1 signaling is critical for the expression of PTN in macrophages.
Figures

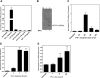
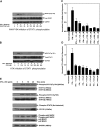


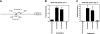

Similar articles
-
IFN-gamma-induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes.Glia. 2007 Feb;55(3):253-62. doi: 10.1002/glia.20451. Glia. 2007. PMID: 17091494
-
Critical role for casein kinase 2 and phosphoinositide-3-kinase in the interferon-gamma-induced expression of monocyte chemoattractant protein-1 and other key genes implicated in atherosclerosis.Arterioscler Thromb Vasc Biol. 2007 Apr;27(4):806-12. doi: 10.1161/01.ATV.0000258867.79411.96. Epub 2007 Jan 25. Arterioscler Thromb Vasc Biol. 2007. PMID: 17255531
-
Interferon-γ biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells.FASEB J. 2012 May;26(5):1821-30. doi: 10.1096/fj.11-195198. Epub 2012 Feb 1. FASEB J. 2012. PMID: 22302831 Free PMC article.
-
Obligatory role of heat shock protein 90 in iNOS induction.Am J Physiol Cell Physiol. 2011 Jul;301(1):C227-33. doi: 10.1152/ajpcell.00493.2010. Epub 2011 Mar 23. Am J Physiol Cell Physiol. 2011. PMID: 21430289 Free PMC article.
-
ERK is integral to the IFN-γ-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages.J Immunol. 2010 Sep 1;185(5):3041-8. doi: 10.4049/jimmunol.1000993. Epub 2010 Jul 30. J Immunol. 2010. PMID: 20675591
Cited by
-
Genes involved in systemic and arterial bed dependent atherosclerosis--Tampere Vascular study.PLoS One. 2012;7(4):e33787. doi: 10.1371/journal.pone.0033787. Epub 2012 Apr 11. PLoS One. 2012. PMID: 22509262 Free PMC article.
-
Elevated expression of pleiotrophin in human hypertrophic scars.J Mol Histol. 2013 Feb;44(1):91-6. doi: 10.1007/s10735-012-9453-8. Epub 2012 Sep 28. J Mol Histol. 2013. PMID: 23054143
-
Scutellarin ameliorates neonatal hypoxic-ischemic encephalopathy associated with GAP43-dependent signaling pathway.Chin Med. 2021 Oct 18;16(1):105. doi: 10.1186/s13020-021-00517-z. Chin Med. 2021. PMID: 34663387 Free PMC article.
-
From top to bottom: midkine and pleiotrophin as emerging players in immune regulation.J Leukoc Biol. 2017 Aug;102(2):277-286. doi: 10.1189/jlb.3MR1116-475R. Epub 2017 Mar 29. J Leukoc Biol. 2017. PMID: 28356350 Free PMC article. Review.
-
Pleiotrophin drives a prometastatic immune niche in breast cancer.J Exp Med. 2023 May 1;220(5):e20220610. doi: 10.1084/jem.20220610. Epub 2023 Feb 24. J Exp Med. 2023. PMID: 36828390 Free PMC article.
References
-
- Hansson G K. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. - PubMed
-
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. - PubMed
-
- Geng Y J, Holm J, Nygren S, Bruzelius M, Stemme S, Hansson G K. Expression of the macrophage scavenger receptor in atheroma: relationship to immune activation and the T-cell cytokine interferon-γ. Arterioscler Thromb Vasc Biol. 1995;15:1995–2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

