Neonatal innate TLR-mediated responses are distinct from those of adults
- PMID: 19917677
- PMCID: PMC4556237
- DOI: 10.4049/jimmunol.0901481
Neonatal innate TLR-mediated responses are distinct from those of adults
Abstract
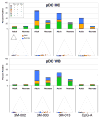
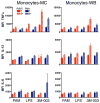
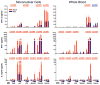
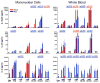
The human neonate and infant are unduly susceptible to infection with a wide variety of microbes. This susceptibility is thought to reflect differences from adults in innate and adaptive immunity, but the nature of these differences is incompletely characterized. The innate immune response directs the subsequent adaptive immune response after integrating information from TLRs and other environmental sensors. We set out to provide a comprehensive analysis defining differences in response to TLR ligation between human neonates and adults. In response to most TLR ligands, neonatal innate immune cells, including monocytes and conventional and plasmacytoid dendritic cells produced less IL-12p70 and IFN-alpha (and consequently induced less IFN-gamma), moderately less TNF-alpha, but as much or even more IL-1beta, IL-6, IL-23, and IL-10 than adult cells. At the single-cell level, neonatal innate cells generally were less capable of producing multiple cytokines simultaneously, i.e., were less polyfunctional. Overall, our data suggest a robust if not enhanced capacity of the neonate vs the adult white-blood cell TLR-mediated response to support Th17- and Th2-type immunity, which promotes defense against extracellular pathogens, but a reduced capacity to support Th1-type responses, which promote defense against intracellular pathogens.
Figures




References
-
- Klein JO, Baker CJ, Remington JS, Wilson CB. Current Concepts of Infections of the Fetus and Newborn Infant. In: Klein JO, Remington JS, Wilson CB, Baker CJ, editors. Infectious Diseases of the Fetus and Newborn Infant. 6. Elsevier Saunders; Philiadelphia: 2006. pp. 1–24.
-
- Lewis DB, Wilson CB. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Remington Jack JOKS, Wilson Christopher B, Baker Carol J., editors. Infectious Diseases of the Fetus and Newborn Infant. 6. Elsevier Saunders; Philiadelphia: 2006. pp. 25–138.
-
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. - PubMed
-
- Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. - PubMed
-
- Joyner JL, Augustine NH, Taylor KA, La Pine TR, Hill HR. Effects of group B streptococci on cord and adult mononuclear cell interleukin-12 and interferon-gamma mRNA accumulation and protein secretion. J Infect Dis. 2000;182:974–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

