CCR5 ligands modulate CXCL12-induced chemotaxis, adhesion, and Akt phosphorylation of human cord blood CD34+ cells
- PMID: 19917679
- PMCID: PMC2818985
- DOI: 10.4049/jimmunol.0900542
CCR5 ligands modulate CXCL12-induced chemotaxis, adhesion, and Akt phosphorylation of human cord blood CD34+ cells
Abstract
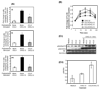
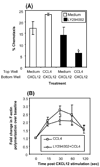
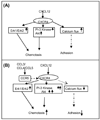
CXCL12 and its receptor CXCR4 play an important role in hematopoietic stem/progenitor cell (HSPC) migration from and retention within the bone marrow. HSPCs are very selective in their chemotactic response and undergo chemotaxis only in response to CXCL12. In addition to CXCR4, HSPCs express receptors for various other chemokines; however, the role of these receptors is not well understood. Freshly isolated CD34(+) cells (highly enriched for HSPCs) from cord blood (CB) express low levels of CCR5; however, if the cells were washed with acidic buffer before Ab staining to remove any ligand bound to CCR5, then nearly 80% of CD34(+) CB cells were found to express CCR5 on the cell surface. Although none of the CCR5 ligands investigated in this study (CCL3, CCL4, and CCL5) induced chemotaxis, at relatively high concentrations they transiently enhanced CXCL12-mediated chemotaxis of CD34(+) CB cells. In contrast, CXCL12-mediated adhesion of cells to VCAM-1-coated surfaces was reduced if CD34(+) CB cells were pretreated with these CCR5 ligands for 15 min. The effect of these chemokines on CXCL12-mediated responses was not at the level of CXCR4 expression, but on downstream signaling pathways elicited by CXCL12. Pretreatment with CCR5 chemokines enhanced CXCL12-mediated Akt phosphorylation, but down-modulated calcium flux in CD34(+) CB cells. Modulation of CXCL12-mediated responses of CD34(+) cells by CCR5 chemokines provides a possible mechanism that underlies movement of HSPCs during inflammation.
Figures





Similar articles
-
Heterologous desensitization of T cell functions by CCR5 and CXCR4 ligands: inhibition of cellular signaling, adhesion and chemotaxis.Int Immunol. 2003 Jan;15(1):29-38. doi: 10.1093/intimm/dxg002. Int Immunol. 2003. PMID: 12502723
-
CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor.Blood. 2002 Oct 1;100(7):2321-9. doi: 10.1182/blood-2002-01-0248. Blood. 2002. PMID: 12239139
-
Virus stimulation of human mast cells results in the recruitment of CD56⁺ T cells by a mechanism dependent on CCR5 ligands.FASEB J. 2012 Mar;26(3):1280-9. doi: 10.1096/fj.11-188979. Epub 2011 Nov 28. FASEB J. 2012. PMID: 22125314
-
Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells.J Immunol. 2002 Dec 15;169(12):7000-8. doi: 10.4049/jimmunol.169.12.7000. J Immunol. 2002. PMID: 12471135
-
[Chemokines and defense-system cell homing].J Soc Biol. 2001;195(1):9-12. J Soc Biol. 2001. PMID: 11530508 Review. French.
Cited by
-
Poly(I:C) primes primary human glioblastoma cells for an immune response invigorated by PD-L1 blockade.Oncoimmunology. 2017 Dec 12;7(3):e1407899. doi: 10.1080/2162402X.2017.1407899. eCollection 2018. Oncoimmunology. 2017. PMID: 29399410 Free PMC article.
-
Glutathione S-transferase P influences redox and migration pathways in bone marrow.PLoS One. 2014 Sep 12;9(9):e107478. doi: 10.1371/journal.pone.0107478. eCollection 2014. PLoS One. 2014. PMID: 25216273 Free PMC article.
-
The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease.Front Physiol. 2014 Jun 11;5:212. doi: 10.3389/fphys.2014.00212. eCollection 2014. Front Physiol. 2014. PMID: 24966838 Free PMC article. Review.
-
The human chorion contains definitive hematopoietic stem cells from the fifteenth week of gestation.Development. 2017 Apr 15;144(8):1399-1411. doi: 10.1242/dev.138438. Epub 2017 Mar 2. Development. 2017. PMID: 28255007 Free PMC article.
-
CXCL12 induces hepatic stellate cell contraction through a calcium-independent pathway.Am J Physiol Gastrointest Liver Physiol. 2013 Sep 1;305(5):G375-82. doi: 10.1152/ajpgi.00185.2012. Epub 2013 Jun 27. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23812037 Free PMC article.
References
-
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. - PubMed
-
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

