Taking advantage: high-affinity B cells in the germinal center have lower death rates, but similar rates of division, compared to low-affinity cells
- PMID: 19917681
- PMCID: PMC4106706
- DOI: 10.4049/jimmunol.0902452
Taking advantage: high-affinity B cells in the germinal center have lower death rates, but similar rates of division, compared to low-affinity cells
Abstract
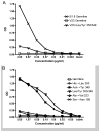
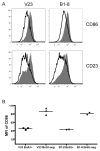
B lymphocytes producing high-affinity Abs are critical for protection from extracellular pathogens, such as bacteria and parasites. The process by which high-affinity B cells are selected during the immune response has never been elucidated. Although it has been shown that high-affinity cells directly outcompete low-affinity cells in the germinal center (GC), whether there are also intrinsic differences between these cells has not been addressed. It could be that higher affinity cells proliferate more rapidly or are more likely to enter cell cycle, thereby outgrowing lower affinity cells. Alternatively, higher affinity cells could be relatively more resistant to cell death in the GC. By comparing high- and low-affinity B cells for the same Ag, we show here that low-affinity cells have an intrinsically higher death rate than do cells of higher affinity, even in the absence of competition. This suggests that selection in the GC reaction is due at least in part to the control of survival of higher affinity B cells and not by a proliferative advantage conferred upon these cells compared with lower affinity B cells. Control over survival rather than proliferation of low- and high-affinity B cells in the GC allows greater diversity not only in the primary response but also in the memory response.
Figures
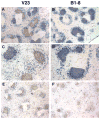
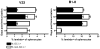
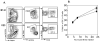


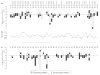

References
-
- Chua MM, Goodgal SH, Karush F. Germ-line affinity and germ-line variable-region genes in the B cell response. J Immunol. 1987;138:1281–1288. - PubMed
-
- Clarke SH, Staudt LM, Kavaler J, Schwartz D, Gerhard WU, Weigert MG. V region gene usage and somatic mutation in the primary and secondary responses to influenza virus hemagglutinin. Journal of Immunology. 1990;144:2795–2801. - PubMed
-
- Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol. 1998;161:5373–5381. - PubMed
-
- Eisen HN, Siskind GW. Variations in Affinities of Antibodies During the Immune Response. Biochemistry. 1964;155:996–1008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

