Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection
- PMID: 19917689
- PMCID: PMC2819141
- DOI: 10.4049/jimmunol.0902446
Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection
Abstract
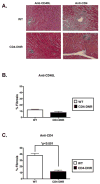
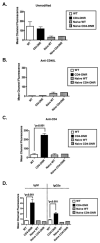
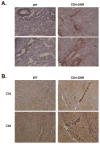
Chronic allograft rejection (CR) is the main barrier to long-term transplant survival. CR is a progressive disease defined by interstitial fibrosis, vascular neointimal development, and graft dysfunction. The underlying mechanisms responsible for CR remain poorly defined. TGFbeta has been implicated in promoting fibrotic diseases including CR, but is beneficial in the transplant setting due to its immunosuppressive activity. To assess the requirement for T cell TGFbeta signaling in allograft acceptance and the progression of CR, we used mice with abrogated T cell TGFbeta signaling as allograft recipients. We compared responses from recipients that were transiently depleted of CD4(+) cells (that develop CR and express intragraft TGFbeta) with responses from mice that received anti-CD40L mAb therapy (that do not develop CR and do not express intragraft TGFbeta). Allograft acceptance and suppression of graft-reactive T and B cells were independent of T cell TGFbeta signaling in mice treated with anti-CD40L mAb. In recipients transiently depleted of CD4(+) T cells, T cell TGFbeta signaling was required for the development of fibrosis associated with CR, long-term graft acceptance, and suppression of graft-reactive T and B cell responses. Furthermore, IL-17 was identified as a critical element in TGFbeta-driven allograft fibrosis. Thus, IL-17 may provide a therapeutic target for preventing graft fibrosis, a measure of CR, while sparing the immunosuppressive activity of TGFbeta.
Conflict of interest statement
Figures




Similar articles
-
TGFbeta neutralization within cardiac allografts by decorin gene transfer attenuates chronic rejection.J Immunol. 2009 Dec 1;183(11):7307-13. doi: 10.4049/jimmunol.0902736. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917705 Free PMC article.
-
Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection.Am J Transplant. 2010 Feb;10(2):220-30. doi: 10.1111/j.1600-6143.2009.02826.x. Epub 2009 Sep 25. Am J Transplant. 2010. PMID: 19788504 Free PMC article.
-
Prevention of acute murine cardiac allograft rejection: anti-CD4 or anti-vascular cell adhesion molecule one monoclonal antibodies block acute rejection but permit persistent graft-reactive alloimmunity and chronic tissue remodelling.J Heart Lung Transplant. 1997 Sep;16(9):889-904. J Heart Lung Transplant. 1997. PMID: 9322138
-
TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection.Immunotherapy. 2010 Jul;2(4):511-20. doi: 10.2217/imt.10.33. Immunotherapy. 2010. PMID: 20636005 Free PMC article. Review.
-
Local cellular immunology of experimental transplant vascular sclerosis.Clin Transplant. 1996 Feb;10(1 Pt 2):100-3. Clin Transplant. 1996. PMID: 8680044 Review.
Cited by
-
Measurement of T Cell Alloreactivity Using Imaging Flow Cytometry.J Vis Exp. 2017 Apr 19;(122):55283. doi: 10.3791/55283. J Vis Exp. 2017. PMID: 28448002 Free PMC article.
-
Induction of TGF-beta 1, not regulatory T cells, impairs antiviral immunity in the lung following bone marrow transplant.J Immunol. 2010 May 1;184(9):5130-40. doi: 10.4049/jimmunol.0901871. Epub 2010 Mar 26. J Immunol. 2010. PMID: 20348421 Free PMC article.
-
The role of IL-17 signaling in regulation of the liver-brain axis and intestinal permeability in Alcoholic Liver Disease.Curr Pathobiol Rep. 2016 Mar;4(1):27-35. doi: 10.1007/s40139-016-0097-3. Epub 2016 Feb 19. Curr Pathobiol Rep. 2016. PMID: 27239399 Free PMC article.
-
Tim-1-Fc suppresses chronic cardiac allograft rejection and vasculopathy by reducing IL-17 production.Int J Clin Exp Pathol. 2014 Jan 15;7(2):509-20. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24551271 Free PMC article.
-
TGF-β-secreting regulatory B cells: unsung players in immune regulation.Clin Transl Immunology. 2021 Apr 2;10(4):e1270. doi: 10.1002/cti2.1270. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33815797 Free PMC article. Review.
References
-
- Waaga AM, Gasser M, Laskowski I, Tilney NL. Mechanisms of chronic rejection. Curr Opin Immunol. 2000;12:517–521. - PubMed
-
- Mannon RB. Therapeutic targets in the treatment of allograft fibrosis. Am J Transplant. 2006;6:867–875. - PubMed
-
- Orosz CG, Pelletier RP. Chronic remodeling pathology in grafts. Curr Opin Immunol. 1997;9:676–680. - PubMed
-
- Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin Nephrol. 2000;20:126–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

