Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer
- PMID: 19917717
- PMCID: PMC2812189
- DOI: 10.1128/IAI.00980-09
Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer
Abstract
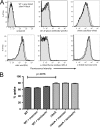
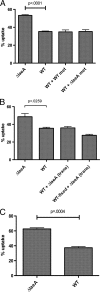
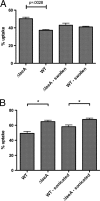
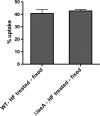
Aspergillus fumigatus is the causal agent of the life-threatening disease invasive aspergillosis. A. fumigatus laeA deletants, aberrant in toxin biosynthesis and spore development, are decreased in virulence. Among other characteristics, the decreased virulence is associated with increased spore susceptibility to macrophage phagocytosis. Three characteristics, cell wall microbe-associated molecular patterns (MAMPs), secreted metabolites, and rodlet content, thought to be important in macrophage-Aspergillus spore interactions were examined. Flow cytometry analysis of wild-type and DeltalaeA spores did not reveal any differences in surface-accessible MAMPs, including beta-(1,3)-glucan, alpha-mannose, chitin, and other carbohydrate ligands. Blocking experiments with laminarin and mannan supported the conclusion that differences in cell wall carbohydrates were not responsible for enhanced DeltalaeA spore phagocytosis. Aspergillus spores have been reported to secrete metabolites affecting phagocytosis. Neither spent culture exchange, transwell, nor coincubation internalization experiments supported a role for secreted metabolites in the differential uptake of wild-type and DeltalaeA spores. However, sonication assays implicated a role for surface rodlet protein/hydrophobin (RodAp) in differential spore phagocytosis. A possible role of RodAp in enhanced DeltalaeA spore uptake was further assessed by RodAp extraction and quantification, where wild-type spores were found to contain 60% more RodAp than DeltalaeA spores. After removal of the surface rodlet layer, wild-type spores were phagocytosed at similar rates as DeltalaeA spores. We conclude that increased uptake of DeltalaeA resting spores is not associated with changes in secreted metabolite production of this mutant or surface carbohydrate availability but, rather, due to a decrease in the surface RodAp content of DeltalaeA spores. We theorize that RodAp acts as an antiphagocytic molecule, possibly via physicochemical means and/or by impeding MAMP recognition by macrophage receptors.
Figures


References
-
- Aimanianda, V., J. Bayry, S. Bozza, O. Kniemeyer, K. Perruccio, S. R. Elluru, C. Clavaud, S. Paris, A. A. Brakhage, S. V. Kaveri, L. Romani, and J.-P. Latgé. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117-1121. - PubMed
-
- Bertout, S., C. Badoc, M. Mallie, J. Giaimis, and J. M. Bastide. 2002. Spore diffusate isolated from some strains of Aspergillus fumigatus inhibits phagocytosis by murine alveolar macrophages. FEMS Immunol. Med. Microbiol. 33:101-106. - PubMed
-
- Brown, G. D., and S. Gordon. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36-37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

