In vitro and in vivo efficacies of teicoplanin-loaded calcium sulfate for treatment of chronic methicillin-resistant Staphylococcus aureus osteomyelitis
- PMID: 19917757
- PMCID: PMC2798490
- DOI: 10.1128/AAC.01122-09
In vitro and in vivo efficacies of teicoplanin-loaded calcium sulfate for treatment of chronic methicillin-resistant Staphylococcus aureus osteomyelitis
Abstract
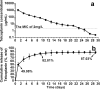
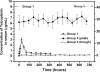
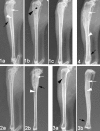
The in vitro and in vivo therapeutic efficacies of teicoplanin-loaded calcium sulfate (TCS; 10% [wt] teicoplanin) were investigated in a rabbit model of chronic methicillin-resistant Staphylococcus aureus (MRSA) osteomyelitis. The in vitro elution characteristics of teicoplanin from TCS pellets were realized by carrying out an evaluation of the release kinetics, recovery rate, and antibacterial activity of the released teicoplanin. Chronic osteomyelitis was induced by inoculating 10(7) CFU of a MRSA strain into the tibial cavity of rabbits. After 3 weeks, the animals were treated by debridement followed by implantation of TCS pellets in group 1, calcium sulfate (CS) pellets alone in group 2, and intravenous (i.v.) teicoplanin (6 mg/kg of body weight every 12 h for three doses and then every 24 h up to 4 weeks) in group 3. Animals in group 4 were left untreated. After 6 weeks, the efficacy of the osteomyelitis treatment was evaluated by hematological, radiological, microbiological, and histological examinations. In vitro elution studies showed sustained release of teicoplanin at a therapeutic level over a time period of 3 weeks. The released teicoplanin maintained its antibacterial activity. In vivo, the best therapeutic effect was observed in animals treated with TCS pellets, resulting in significantly lower radiological and histological scores, lower positive rates of MRSA culture and bacterial load, and excellent bone regeneration compared with those treated by CS alone or i.v. teicoplanin, without any local or systemic adverse effects. TCS pellets are an effective alternative to i.v. teicoplanin for the treatment of chronic MRSA osteomyelitis, particularly because teicoplanin is delivered locally while the TCS pellets simultaneously promote bone defect repair.
Figures




Similar articles
-
Comparison of Borate Bioactive Glass and Calcium Sulfate as Implants for the Local Delivery of Teicoplanin in the Treatment of Methicillin-Resistant Staphylococcus aureus-Induced Osteomyelitis in a Rabbit Model.Antimicrob Agents Chemother. 2015 Dec;59(12):7571-80. doi: 10.1128/AAC.00196-15. Epub 2015 Sep 28. Antimicrob Agents Chemother. 2015. PMID: 26416858 Free PMC article.
-
Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis.Acta Biomater. 2010 Mar;6(3):812-9. doi: 10.1016/j.actbio.2009.09.011. Epub 2009 Sep 19. Acta Biomater. 2010. PMID: 19770078
-
Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model.Biomaterials. 2010 Aug;31(22):5865-74. doi: 10.1016/j.biomaterials.2010.04.005. Biomaterials. 2010. PMID: 20434766
-
Optimal trough concentration of teicoplanin for the treatment of methicillin-resistant Staphylococcus aureus infection: A systematic review and meta-analysis.J Clin Pharm Ther. 2021 Jun;46(3):622-632. doi: 10.1111/jcpt.13366. Epub 2021 Feb 6. J Clin Pharm Ther. 2021. PMID: 33547647
-
Osteomyelitis: Focus on Conventional Treatments and Innovative Drug Delivery Systems.Curr Drug Deliv. 2021;18(5):532-545. doi: 10.2174/1567201817666200915093224. Curr Drug Deliv. 2021. PMID: 32933461 Review.
Cited by
-
Effects of Local Application of Nano-silver on Osteomyelitis and Soft Tissue Infections: An Experimental Study in Rats.J Bone Jt Infect. 2018 Apr 12;3(1):43-49. doi: 10.7150/jbji.22121. eCollection 2018. J Bone Jt Infect. 2018. PMID: 29774178 Free PMC article.
-
Chronic Osteomyelitis - Bacterial Flora, Antibiotic Sensitivity and Treatment Challenges.Open Orthop J. 2018 Mar 30;12:153-163. doi: 10.2174/1874325001812010153. eCollection 2018. Open Orthop J. 2018. PMID: 29755606 Free PMC article.
-
Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis.Crit Rev Ther Drug Carrier Syst. 2015;32(1):1-59. doi: 10.1615/critrevtherdrugcarriersyst.2014010920. Crit Rev Ther Drug Carrier Syst. 2015. PMID: 25746204 Free PMC article. Review.
-
Chitosan sponges for local synergistic infection therapy: a pilot study.Clin Orthop Relat Res. 2013 Oct;471(10):3158-64. doi: 10.1007/s11999-013-2988-5. Epub 2013 Apr 20. Clin Orthop Relat Res. 2013. PMID: 23604649 Free PMC article.
-
Dual delivery of active antibactericidal agents and bone morphogenetic protein at sustainable high concentrations using biodegradable sheath-core-structured drug-eluting nanofibers.Int J Nanomedicine. 2016 Aug 17;11:3927-37. doi: 10.2147/IJN.S107250. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27574423 Free PMC article.
References
-
- Antoci, V., Jr., C. S. Adams, N. J. Hickok, I. M. Shapiro, and J. Parvizi. 2007. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 462:200-206. - PubMed
-
- Brogden, R. N., and D. H. Peters. 1994. Teicoplanin. A reappraisal of its antimicrobial activity, pharmacokinetic properties therapeutic efficacy. Drugs 47:823-854. - PubMed
-
- Cepeda, J. A., T. Whitehouse, B. Cooper, J. Hails, K. Jones, F. Kwaku, L. Taylor, S. Hayman, S. Shaw, C. Kibbler, R. Shulman, M. Singer, and A. P. Wilson. 2004. Linezolid versus teicoplanin in the treatment of Gram-positive infections in the critically ill: a randomized, double-blind, multicentre study. J. Antimicrob. Chemother. 53:345-355. - PubMed
-
- Chang, W., M. Colangeli, S. Colangeli, C. Di Bella, E. Gozzi, and D. Donati. 2007. Adult osteomyelitis: debridement versus debridement plus Osteoset T pellets. Acta Orthop. Belg. 73:238-243. - PubMed
-
- Courvalin, P. 2006. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 42:S25-S34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

