Structure-microbicidal activity relationship of synthetic fragments derived from the antibacterial alpha-helix of human lactoferrin
- PMID: 19917761
- PMCID: PMC2798562
- DOI: 10.1128/AAC.00908-09
Structure-microbicidal activity relationship of synthetic fragments derived from the antibacterial alpha-helix of human lactoferrin
Abstract
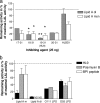
There is a need for new microbicidal agents with therapeutic potential due to antibiotic resistance in bacteria and fungi. In this study, the structure-microbicidal activity relationship of amino acid residues 14 to 31 (sequence 14-31) from the N-terminal end, corresponding to the antibacterial alpha-helix of human lactoferrin (LF), was investigated by downsizing, alanine scanning, and substitution of amino acids. Microbicidal analysis (99% killing) was performed by a microplate assay using Escherichia coli, Staphylococcus aureus, and Candida albicans as test organisms. Starting from the N-terminal end, downsizing of peptide sequence 14-31 showed that the peptide sequence 19-31 (KCFQWQRNMRKVR, HL9) was the optimal length for antimicrobial activity. Furthermore, HL9 bound to lipid A/lipopolysaccharide, as shown by neutralizing endotoxic activity in a Limulus assay. Alanine scanning of peptide sequence 20-31 showed that Cys20, Trp23, Arg28, Lys29, or Arg31 was important for expressing full killing activity, particularly against C. albicans. Substituting the neutral hydrophilic amino acids Gln24 and Asn26 for Lys and Ala (HLopt2), respectively, enhanced microbicidal activity significantly against all test organisms compared to the amino acids natural counterpart, also, in comparison with HL9, HLopt2 had more than 10-fold-stronger fungicidal activity. Furthermore, HLopt2 was less affected by metallic salts than HL9. The microbicidal activity of HLopt2 was slightly reduced only at pH 7.0, as tested in the pH range of 4.5 to 7.5. The results showed that the microbicidal activity of synthetic peptide sequences, based on the antimicrobial alpha-helix region of LF, can be significantly enhanced by optimizing the length and substitution of neutral amino acids at specific positions, thus suggesting a sequence lead with therapeutic potential.
Figures



Similar articles
-
Antibiotic-Based Conjugates Containing Antimicrobial HLopt2 Peptide: Design, Synthesis, Antimicrobial and Cytotoxic Activities.ACS Chem Biol. 2019 Oct 18;14(10):2233-2242. doi: 10.1021/acschembio.9b00538. Epub 2019 Sep 25. ACS Chem Biol. 2019. PMID: 31513374
-
Antibacterial activity of 15-residue lactoferricin derivatives.J Pept Res. 2000 Nov;56(5):265-74. doi: 10.1034/j.1399-3011.2000.00770.x. J Pept Res. 2000. PMID: 11095180
-
Antibacterial activity of peptides homologous to a loop region in human lactoferrin.FEBS Lett. 1996 Mar 11;382(1-2):175-8. doi: 10.1016/0014-5793(96)00168-8. FEBS Lett. 1996. PMID: 8612745
-
Lactoferricin derived from milk protein lactoferrin.Curr Pharm Des. 2003;9(16):1277-87. doi: 10.2174/1381612033454829. Curr Pharm Des. 2003. PMID: 12769736 Review.
-
Microbicidal activity of MDI-P against Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa, and Legionella pneumophila.Am J Infect Control. 2000 Jun;28(3):251-7. doi: 10.1067/mic.2000.105287. Am J Infect Control. 2000. PMID: 10840346 Review.
Cited by
-
Mimicking Nonribosomal Peptides from the Marine Actinomycete Streptomyces sp. H-KF8 Leads to Antimicrobial Peptides.ACS Infect Dis. 2024 Jan 12;10(1):79-92. doi: 10.1021/acsinfecdis.3c00206. Epub 2023 Dec 19. ACS Infect Dis. 2024. PMID: 38113038 Free PMC article.
-
The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens.Front Microbiol. 2017 Jan 18;8:2. doi: 10.3389/fmicb.2017.00002. eCollection 2017. Front Microbiol. 2017. PMID: 28149293 Free PMC article. Review.
-
Peptides Released from Foremilk and Hindmilk Proteins by Breast Milk Proteases Are Highly Similar.Front Nutr. 2017 Nov 2;4:54. doi: 10.3389/fnut.2017.00054. eCollection 2017. Front Nutr. 2017. PMID: 29164128 Free PMC article.
-
Characterization of the in vitro, ex vivo, and in vivo Efficacy of the Antimicrobial Peptide DPK-060 Used for Topical Treatment.Front Cell Infect Microbiol. 2019 May 28;9:174. doi: 10.3389/fcimb.2019.00174. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31192163 Free PMC article.
-
Anti-infectious and anti-inflammatory effects of peptide fragments sequentially derived from the antimicrobial peptide centrocin 1 isolated from the green sea urchin, Strongylocentrotus droebachiensis.AMB Express. 2012 Dec 13;2(1):67. doi: 10.1186/2191-0855-2-67. AMB Express. 2012. PMID: 23237525 Free PMC article.
References
-
- Aguilera, O., H. Ostolaza, L. M. Quiros, and J. F. Fierro. 1999. Permeabilizing action of an antimicrobial lactoferricin-derived peptide on bacterial and artificial membranes. FEBS Lett. 462:273-277. - PubMed
-
- Anderson, B. F., H. M. Baker, G. E. Norris, D. W. Rice, and E. N. Baker. 1989. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 Å resolution. J. Mol. Biol. 209:711-734. - PubMed
-
- Bellamy, W., M. Takase, K. Yamauchi, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130-136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

