Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells
- PMID: 19917776
- PMCID: PMC2806625
- DOI: 10.1084/jem.20091472
Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells
Abstract
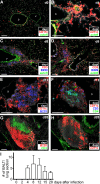
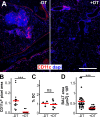
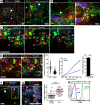
Mucosal vaccination via the respiratory tract can elicit protective immunity in animal infection models, but the underlying mechanisms are still poorly understood. We show that a single intranasal application of the replication-deficient modified vaccinia virus Ankara, which is widely used as a recombinant vaccination vector, results in prominent induction of bronchus-associated lymphoid tissue (BALT). Although initial peribronchiolar infiltrations, characterized by the presence of dendritic cells (DCs) and few lymphocytes, can be found 4 d after virus application, organized lymphoid structures with segregated B and T cell zones are first observed at day 8. After intratracheal application, in vitro-differentiated, antigen-loaded DCs rapidly migrate into preformed BALT and efficiently activate antigen-specific T cells, as revealed by two-photon microscopy. Furthermore, the lung-specific depletion of DCs in mice that express the diphtheria toxin receptor under the control of the CD11c promoter interferes with BALT maintenance. Collectively, these data identify BALT as tertiary lymphoid structures supporting the efficient priming of T cell responses directed against unrelated airborne antigens while crucially requiring DCs for its sustained presence.
Figures


References
-
- Corbett M., Bogers W.M., Heeney J.L., Gerber S., Genin C., Didierlaurent A., Oostermeijer H., Dubbes R., Braskamp G., Lerondel S., et al. 2008. Aerosol immunization with NYVAC and MVA vectored vaccines is safe, simple, and immunogenic. Proc. Natl. Acad. Sci. USA. 105:2046–2051 10.1073/pnas.0705191105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

