Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells
- PMID: 19917779
- PMCID: PMC2794233
- DOI: 10.1681/ASN.2009010122
Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells
Abstract
Cellular lesions form in Bowman's space in both crescentic glomerulonephritis and collapsing glomerulopathy. The pathomechanism and origin of the proliferating cells in these lesions are unknown. In this study, we examined proliferating cells by lineage tracing of either podocytes or parietal epithelial cells (PECs) in the nephrotoxic nephritis model of inflammatory crescentic glomerulonephritis. In addition, we traced the fate of genetically labeled PECs in the Thy-1.1 transgenic mouse model of collapsing glomerulopathy. In both models, cellular bridges composed of PECs were observed between Bowman's capsule and the glomerular tuft. Genetically labeled PECs also populated larger, more advanced cellular lesions. In these lesions, we detected de novo expression of CD44 in activated PECs. In contrast, we rarely identified genetically labeled podocytes within the cellular lesions of crescentic glomerulonephritis. In conclusion, PECs constitute the majority of cells that compose early extracapillary proliferative lesions in both crescentic glomerulonephritis and collapsing glomerulopathy, suggesting similar pathomechanisms in both diseases.
Figures
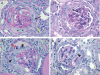
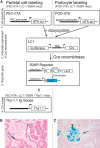
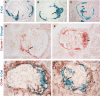
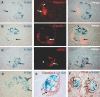
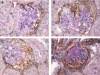
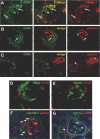
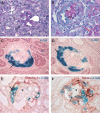
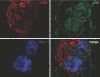
References
-
- Jennette JC, Olson JL, Schwartz MM, Silva FG: Crescentic glomerulonephritis. In: Heptinstall's Pathology of the Kidney, 6th Ed., Philadelphia, Lippincott-Raven, pp 615–641, 2005
-
- Le Hir M, Besse-Eschmann V: A novel mechanism of nephron loss in a murine model of crescentic glomerulonephritis. Kidney Int 63: 591–599, 2003 - PubMed
-
- Detwiler RK, Falk RJ, Hogan SL, Jennette JC: Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int 45: 1416–1424, 1994 - PubMed
-
- D'Agati V, Suh JI, Carbone L, Cheng JT, Appel G: Pathology of HIV-associated nephropathy: A detailed morphologic and comparative study. Kidney Int 35: 1358–1370, 1989 - PubMed
-
- Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH, Jordan SC, Toyoda M: Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 59: 2126–2133, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

