Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909
- PMID: 19917845
- PMCID: PMC2793032
- DOI: 10.1200/JCO.2009.22.2554
Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909
Abstract
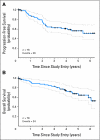
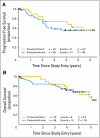
PURPOSE Mantle-cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin's lymphoma with a poor prognosis. We explored the feasibility, safety, and effectiveness of an aggressive immunochemotherapy treatment program that included autologous stem-cell transplantation (ASCT) for patients up to age 69 years with newly diagnosed MCL. PATIENTS AND METHODS The primary end point was 2-year progression-free survival (PFS). A successful trial would yield a 2-year PFS of at least 50% and an event rate (early progression plus nonrelapse mortality) less than 20% at day +100 following ASCT. Seventy-eight patients were treated with two or three cycles of rituximab combined with methotrexate and augmented CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). This treatment was followed by intensification with high doses of cytarabine and etoposide combined with rituximab and filgrastim to mobilize autologous peripheral-blood stem cells. Patients then received high doses of carmustine, etoposide, and cyclophosphamide followed by ASCT and two doses of rituximab. Results There were two nonrelapse mortalities, neither during ASCT. With a median follow-up of 4.7 years, the 2-year PFS was 76% (95% CI, 64% to 85%), and the 5-year PFS was 56% (95% CI, 43% to 68%). The 5-year overall survival was 64% (95% CI, 50% to 75%). The event rate by day +100 of ASCT was 5.1%. CONCLUSION The Cancer and Leukemia Group B 59909 regimen is feasible, safe, and effective in patients with newly diagnosed MCL. The incorporation of rituximab with aggressive chemotherapy and ASCT may be responsible for the encouraging outcomes demonstrated in this study, which produced results comparable to similar treatment regimens.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23:6409–6414. - PubMed
-
- Brody J, Advani R. Treatment of mantle cell lymphoma: Current approach and future directions. Crit Rev Oncol Hematol. 2006;58:257–265. - PubMed
-
- Andersen NS, Jensen MK, de Nully Brown P, et al. A Danish population-based analysis of 105 mantle cell lymphoma patients: Incidences, clinical features, response, and prognostic factors. Eur J Cancer. 2002;38:401–408. - PubMed
-
- Fernàndez V, Hartmann E, Ott G, et al. Pathogenesis of mantle-cell lymphoma: All oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–6369. - PubMed
-
- Bertoni F, Rinaldi A, Zucca E, et al. Update on the molecular biology of mantle cell lymphoma. Hematol Oncol. 2006;24:22–27. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U10 CA032291/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- CA41287/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA077597/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- CA77440/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- CA77658/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- CA77597/CA/NCI NIH HHS/United States
- CA11789/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- CA03927/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA003927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

