Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway
- PMID: 19917848
- PMCID: PMC4288616
- DOI: 10.1200/JCO.2008.21.5988
Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway
Abstract
Purpose: We investigated the global gene expression in a large panel of pancreatic endocrine tumors (PETs) aimed at identifying new potential targets for therapy and biomarkers to predict patient outcome.
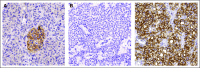
Patients and methods: Using a custom microarray, we analyzed 72 primary PETs, seven matched metastases, and 10 normal pancreatic samples. Relevant differentially expressed genes were validated by either quantitative real-time polymerase chain reaction or immunohistochemistry on tissue microarrays.
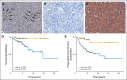
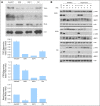
Results: Our data showed that: tuberous sclerosis 2 (TSC2) and phosphatase and tensin homolog (PTEN) were downregulated in most of the primary tumors, and their low expression was significantly associated with shorter disease-free and overall survival; somatostatin receptor 2 (SSTR2) was absent or very low in insulinomas compared with nonfunctioning tumors; and expression of fibroblast growth factor 13 (FGF13) gene was significantly associated with the occurrence of liver metastasis and shorter disease-free survival. TSC2 and PTEN are two key inhibitors of the Akt/mammalian target of rapamycin (mTOR) pathway and the specific inhibition of mTOR with rapamycin or RAD001 inhibited cell proliferation of PET cell lines.
Conclusion: Our results strongly support a role for PI3K/Akt/mTOR pathway in PET, which ties in with the fact that mTOR inhibitors have reached phase III trials in neuroendocrine tumors. The finding of differential SSTR expression raises the potential for SSTR expression to be evaluated as a marker of response to somatostatin analogs. Finally, we identified FGF13 as a new prognostic marker that predicted poorer outcome in patients who were clinically considered free from disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



References
-
- Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: The WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. - PubMed
-
- Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19:903–908. - PubMed
-
- Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256–265. - PubMed
-
- Fazio N, de Braud F, Delle Fave G, et al. Interferon-alpha and somatostatin analog in patients with gastroenteropancreatic neuroendocrine carcinoma: Single agent or combination? Ann Oncol. 2007;18:13–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

