HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells
- PMID: 19918062
- PMCID: PMC2777969
- DOI: 10.1073/pnas.0911742106
HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells
Abstract
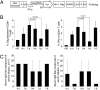
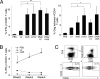
Lentivectors (LVs) have attracted considerable interest for their potential as a vaccine delivery vehicle. In this study, we evaluate in mice a dendritic cell (DC)-directed LV system encoding the Gag protein of human immunodeficiency virus (HIV) (LV-Gag) as a potential vaccine for inducing an anti-HIV immune response. The DC-directed specificity is achieved through pseudotyping the vector with an engineered Sindbis virus glycoprotein capable of selectively binding to the DC-SIGN protein. A single immunization by this vector induces a durable HIV Gag-specific immune response. We investigated the antigen-specific immunity and T-cell memory generated by a prime/boost vaccine regimen delivered by either successive LV-Gag injections or a DNA prime/LV-Gag boost protocol. We found that both prime/boost regimens significantly enhance cellular and humoral immune responses. Importantly, a heterologous DNA prime/LV-Gag boost regimen results in superior Gag-specific T-cell responses as compared with a DNA prime/adenovector boost immunization. It induces not only a higher magnitude response, as measured by Gag-specific tetramer analysis and intracellular IFN-gamma staining, but also a better quality of response evidenced by a wider mix of cytokines produced by the Gag-specific CD8(+) and CD4(+) T cells. A boosting immunization with LV-Gag also generates T cells reactive to a broader range of Gag-derived epitopes. These results demonstrate that this DC-directed LV immunization is a potent modality for eliciting anti-HIV immune responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

