Fibulin-5 contributes to microfibril assembly in human periodontal ligament cells
- PMID: 19918324
- PMCID: PMC2775106
- DOI: 10.1267/ahc.09021
Fibulin-5 contributes to microfibril assembly in human periodontal ligament cells
Abstract
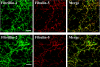
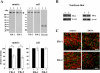
The elastic system fibers comprise oxytalan, elaunin and elastic fibers, which differ in their relative microfibril and elastin content. Human periodontal ligaments (PDL) contain only oxytalan fibers (pure microfibrils) among them. Since fibulin-5 regulates the organization of elastic fibers to link the fibers to cells, we hypothesized that fibulin-5 may contribute to the formation of oxytalan fibers. We used siRNA for fibulin-5 in PDL cell culture to examine the extracellular deposition of fibrillin-1 and -2, which are the major components of microfibrils. Fibulin-5 was labeled on microfibrils positive for fibrillin-1 and -2. Fibulin-5 suppression reduced the level of fibrillin-1 and -2 deposition to 60% of the control level. These results suggest that fibulin-5 may control the formation of oxytalan fibers, and play a role in the homeostasis of oxytalan fibers.
Keywords: fibrillin; fibulin-5; microfibrils; oxytalan fiber; periodontal ligaments.
Figures
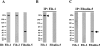

Similar articles
-
The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament.Cells. 2025 May 22;14(11):764. doi: 10.3390/cells14110764. Cells. 2025. PMID: 40497939 Free PMC article. Review.
-
Stretching stimulates fibulin-5 expression and controls microfibril bundles in human periodontal ligament cells.J Periodontal Res. 2009 Oct;44(5):622-7. doi: 10.1111/j.1600-0765.2008.01170.x. Epub 2008 Oct 22. J Periodontal Res. 2009. PMID: 18973521
-
EMILIN-1 regulates the amount of oxytalan fiber formation in periodontal ligaments in vitro.Connect Tissue Res. 2011 Feb;52(1):30-5. doi: 10.3109/03008207.2010.502982. Epub 2010 Aug 11. Connect Tissue Res. 2011. PMID: 20701466
-
Stretching modulates oxytalan fibers in human periodontal ligament cells.J Periodontal Res. 2009 Apr;44(2):170-4. doi: 10.1111/j.1600-0765.2008.01099.x. Epub 2008 Jun 17. J Periodontal Res. 2009. PMID: 18565133
-
Microfibrils: a cornerstone of extracellular matrix and a key to understand Marfan syndrome.Ital J Anat Embryol. 2009 Oct-Dec;114(4):201-24. Ital J Anat Embryol. 2009. PMID: 20578676 Review.
Cited by
-
Fibulin-5 expression in the human placenta.Histochem Cell Biol. 2011 Feb;135(2):203-13. doi: 10.1007/s00418-011-0784-4. Epub 2011 Feb 3. Histochem Cell Biol. 2011. PMID: 21290250
-
Heat shock protein 47: a novel biomarker of phenotypically altered collagen-producing cells.Acta Histochem Cytochem. 2011 Apr 28;44(2):35-41. doi: 10.1267/ahc.11001. Epub 2011 Apr 21. Acta Histochem Cytochem. 2011. PMID: 21614164 Free PMC article.
-
Single Cell RNA Sequencing Reveals Critical Functions of Mkx in Periodontal Ligament Homeostasis.Front Cell Dev Biol. 2022 Feb 4;10:795441. doi: 10.3389/fcell.2022.795441. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35186919 Free PMC article.
-
Fibulin-4 and -5, but not Fibulin-2, are Associated with Tropoelastin Deposition in Elastin-Producing Cell Culture.Acta Histochem Cytochem. 2010 Dec 29;43(6):131-8. doi: 10.1267/ahc.10026. Epub 2010 Nov 20. Acta Histochem Cytochem. 2010. PMID: 21245979 Free PMC article.
-
The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament.Cells. 2025 May 22;14(11):764. doi: 10.3390/cells14110764. Cells. 2025. PMID: 40497939 Free PMC article. Review.
References
-
- Carta L., Pereira L., Arteaga-Solis E., Lee-Arteaga S. Y., Lenart B., Starcher B., Merkel C. A., Sukoyan M., Kerkis A., Hazeki N., Keene D. R., Sakai L. Y., Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006;281:8016–8023. - PMC - PubMed
-
- Charbonneau N. L., Dzamba B. J., Ono R. N., Keene D. R., Corson G. M., Reinhardt D. P., Sakai L. Y. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J. Biol. Chem. 2003;278:2740–2749. - PubMed
-
- Chavrier C., Hartmann D. J., Couble M. L., Herbage D. Distribution and organization of the elastic system fibres in healthy human gingiva. Ultrastructural and immunohistochemical study. Histochemistry. 1988;89:47–52. - PubMed

