Water-soluble fullerene (C60) inhibits the development of arthritis in the rat model of arthritis
- PMID: 19918368
- PMCID: PMC2775692
- DOI: 10.2147/ijn.s7653
Water-soluble fullerene (C60) inhibits the development of arthritis in the rat model of arthritis
Abstract
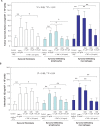
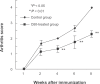
Recently, it has been demonstrated that oxygen free radicals have an important role as a signaling messenger in the development of inflammation and osteoclastogenesis, suggesting the implication of oxygen free radicals in the pathogenesis of arthritis. The aim of this study was to examine the potential of a strong free-radical scavenger, water-soluble fullerene (C60), as a protective agent against synovitis in arthritis, both in vitro and in vivo. In the presence or absence of C60 (0.1, 1.0, 10.0 muM), human synovial fibroblasts, synovial infiltrating lymphocytes or macrophages were incubated with tumor necrosis factor-alpha (TNF-alpha) (10.0 ng/mL), and the production of proinflammatory cytokines by the individual cells were analyzed. C60 significantly suppressed the TNF-alpha-induced production of proinflammatory cytokines in synovial fibroblasts, synovial infiltrating lymphocytes and macrophages in vitro. Adjuvant induced arthritic rats were used as an animal model of arthritis. Rats were divided into two subgroups: control and treatment with C60 at 10.0 muM. The left ankle joint was injected intraarticularly with water-soluble C60 (20 mul) in the C60-treated group, while, as a control, the left ankle joint in the control rats received phosphate-buffered saline (20 mul), once weekly for eight weeks. Ankle joint tissues were prepared for histological analysis. In adjuvant-induced arthritic rats, intra-articular treatment with C60 in vivo reduced synovitis and alleviated bone resorption and destruction in the joints, while control ankle joints showed progression of synovitis and joint destruction with time. These findings indicate that C60 is a potential therapeutic agent for inhibition of arthritis.
Keywords: arthritis; bone resorption; fullerene; inflammation; synovitis.
Figures



References
-
- Rooney M, Condell D, Quinlan W, et al. Analysis of the histological variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988;31:956–963. - PubMed
-
- Cush JJ, Pietschmann P, Oppenheimer-Marks N, Lipsky PE. The intrinsic migratory capacity of memory T cells contributes to their accumulation in rheumatoid synovium. Arthritis Rheum. 1992;35:1434–1444. - PubMed
-
- Firestein GS. Rheumatoid synovitis and pannus. In: Klippel JH, Dieppe PA, editors. Rheumatology. London, UK: Mosby; 1998. pp. 1–24.
-
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–2895. - PubMed
-
- Tambar S, Ruderman EM. Current management of rheumatoid arthritis. Manag Care Interface. 2007;20(7):18–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

