HYDROGELS FROM SOFT CONTACT LENSES AND IMPLANTS TO SELF-ASSEMBLED NANOMATERIALS
- PMID: 19918374
- PMCID: PMC2776732
- DOI: 10.1002/pola.23607
HYDROGELS FROM SOFT CONTACT LENSES AND IMPLANTS TO SELF-ASSEMBLED NANOMATERIALS
Abstract
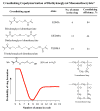
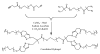
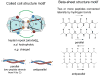
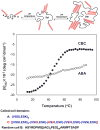
Hydrogels were the first biomaterials designed for clinical use. Their discovery and applications as soft contact lenses and implants are presented. This early hydrogel research served as a foundation for the expansion of biomedical polymers research into new directions: design of stimuli sensitive hydrogels that abruptly change their properties upon application of an external stimulus (pH, temperature, solvent, electrical field, biorecognition) and hydrogels as carriers for the delivery of drugs, peptides, and proteins. Finally, pathways to self-assembly of block and graft copolymers into hydrogels of precise 3D structures are introduced.
Figures




References
-
- Kopeček J, Yang J. Polymer Int. 2007;56:1078–1098.
-
- Dušek K, Prins W. Adv Polym Sci. 1969;6:1–102.
-
- Wichterle O. Encyclopedia of Polymer Science and Technology. In: Mark HF, Gaylord NG, Bikales N, editors. Interscience. Vol. 15. New York, NY: 1971. pp. 273–291.
-
- Andrade JD, editor. Hydrogels for Medical and Related Applications. ACS Symposium Series. Vol. 31. Washington D.C: 1976.
-
- Ratner BD, Hoffman A. Hydrogels for Medical and Related Applications. In: Andrade JD, editor. ACS Symposium Series. Vol. 31. Washington D.C: 1976. pp. 1–36.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
