RF shimming for spectroscopic localization in the human brain at 7 T
- PMID: 19918903
- PMCID: PMC2811270
- DOI: 10.1002/mrm.22182
RF shimming for spectroscopic localization in the human brain at 7 T
Abstract
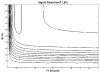
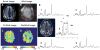
Spectroscopic imaging of the human head at short echo times (<or=15 ms) typically requires suppression of signals from extracerebral tissues. However, at 7 T, decreasing efficiency in B1+ generation (hertz/watt) and increasing spectral bandwidth result in dramatic increases in power deposition and increased chemical shift registration artifacts for conventional gradient-based in-plane localization. In this work, we describe a novel method using radiofrequency shimming and an eight-element transceiver array to generate a B1+ field distribution that excites a ring about the periphery of the head and leaves central brain regions largely unaffected. We have used this novel B1+ distribution to provide in-plane outer volume suppression (>98% suppression of extracerebral lipids) without the use of gradients. This novel B1+ distribution is used in conjunction with a double inversion recovery method to provide suppression of extracerebral resonances with T1s greater than 400 ms, while having negligible effect on metabolite ratios of cerebral resonances with T1s>1000 ms. Despite the use of two adiabatic pulses, the high efficiency of the ring distribution allows radiofrequency power deposition to be limited to 3-4 W for a pulse repetition time of 1.5 sec. The short echo time enabled the acquisition of images of the human brain, displaying glutamate, glutamine, macromolecules, and other major cerebral metabolites.
Copyright (c) 2009 Wiley-Liss, Inc.
Figures








References
-
- Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46(1):24–30. - PubMed
-
- Robitaille PM, Abduljalil AM, Kangarlu A, Zhang X, Yu Y, Burgess R, Bair S, Noa P, Yang L, Zhu H, Palmer B, Jiang Z, Chakeres DM, Spigos D. Human magnetic resonance imaging at 8 T. NMR Biomed. 1998;11(6):263–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

