Recombinant human bone morphogenetic protein-7 enhances fracture healing in an ischemic environment
- PMID: 19918910
- PMCID: PMC2845727
- DOI: 10.1002/jor.21033
Recombinant human bone morphogenetic protein-7 enhances fracture healing in an ischemic environment
Abstract
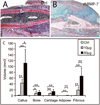
Ischemia predisposes orthopedic trauma patients to delayed fracture healing or nonunion. The goal of this study was to test the ability of bone morphogenetic protein 7 (BMP7) to stimulate fracture repair in an ischemic environment. Ischemic fractures were generated in male adult mice by resecting the femoral artery prior to the creation of a nonstabilized tibia fracture. Recombinant human BMP7 (rhBMP7, 50 microg) was injected into the fracture site immediately after surgery. At 7 days after injury, more tissue vascularization was observed in rhBMP7 treated fractures. Histomorphometric analyses revealed that rhBMP7 induced more cartilage at day 7, more callus and bone at days 14 and 28, and more adipose tissue and fibrous tissue at days 7, 14, and 28 compared to controls (n=5/group/time). At day 28, all fractures treated with rhBMP7 (50 microg, n=5) healed, whereas only three of five control fractures exhibited slight bony bridging. In addition, we found that rhBMP7 (both 10 and 50 microg) significantly increased the amount of cartilage compared to controls in stabilized fractures, confirming its chondrogenic effect. Lastly, using bone marrow transplantation, we determined that no donor-derived osteocytes or chondrocytes were present in rhBMP7-treated fractures, suggesting rhBMP7 did not recruit mesenchymal stem cells from the bone marrow to the fracture site. In conclusion, our results indicate that rhBMP7 is a promising treatment for fractures with severely disrupted blood supply.
Copyright (c) 2009 Orthopaedic Research Society.
Figures





References
-
- Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemp Orthop. 1995;30:489–493. - PubMed
-
- Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–956. - PubMed
-
- Hak DJ, Makino T, Niikura T, et al. Recombinant human BMP-7 effectively prevents nonunion in both young and old rats. J Orthop Res. 2006;24:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

