Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons
- PMID: 19919721
- PMCID: PMC2784755
- DOI: 10.1186/1744-8069-5-65
Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons
Abstract
Background: Neurons in superficial (SDH) and deep (DDH) laminae of the spinal cord dorsal horn receive sensory information from skin, muscle, joints and viscera. In both regions, glycine- (GlyR) and GABAA-receptors (GABAARs) contribute to fast synaptic inhibition. For rat, several types of GABAAR coexist in the two regions and each receptor type provides different contributions to inhibitory tone. Recent work in mouse has discovered an additional type of GlyR, (containing alpha 3 subunits) in the SDH. The contribution of differing forms of the GlyR to sensory processing in SDH and DDH is not understood.
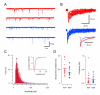
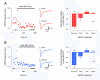
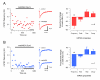
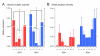
Methods and results: Here we compare fast inhibitory synaptic transmission in mouse (P17-37) SDH and DDH using patch-clamp electrophysiology in transverse spinal cord slices (L3-L5 segments, 23 degrees C). GlyR-mediated mIPSCs were detected in 74% (25/34) and 94% (25/27) of SDH and DDH neurons, respectively. In contrast, GABAAR-mediated mIPSCs were detected in virtually all neurons in both regions (93%, 14/15 and 100%, 18/18). Several Gly- and GABAAR properties also differed in SDH vs. DDH. GlyR-mediated mIPSC amplitude was smaller (37.1 +/- 3.9 vs. 64.7 +/- 5.0 pA; n = 25 each), decay time was slower (8.5 +/- 0.8 vs. 5.5 +/- 0.3 ms), and frequency was lower (0.15 +/- 0.03 vs. 0.72 +/- 0.13 Hz) in SDH vs. DDH neurons. In contrast, GABAAR-mediated mIPSCs had similar amplitudes (25.6 +/- 2.4, n = 14 vs. 25. +/- 2.0 pA, n = 18) and frequencies (0.21 +/- 0.08 vs. 0.18 +/- 0.04 Hz) in both regions; however, decay times were slower (23.0 +/- 3.2 vs. 18.9 +/- 1.8 ms) in SDH neurons. Mean single channel conductance underlying mIPSCs was identical for GlyRs (54.3 +/- 1.6 pS, n = 11 vs. 55.7 +/- 1.8, n = 8) and GABAARs (22.7 +/- 1.7 pS, n = 10 vs. 22.4 +/- 2.0 pS, n = 11) in both regions. We also tested whether the synthetic endocanabinoid, methandamide (methAEA), had direct effects on Gly- and GABAARs in each spinal cord region. MethAEA (5 muM) reduced GlyR-mediated mIPSC frequency in SDH and DDH, but did not affect other properties. Similar results were observed for GABAAR mediated mIPSCs, however, rise time was slowed by methAEA in SDH neurons.
Conclusion: Together these data show that Gly- and GABAARs with clearly differing physiological properties and cannabinoid-sensitivity contribute to fast synaptic inhibition in mouse SDH and DDH.
Figures

References
-
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 3. New York: Kluwer Academic/Plenum Publishers; 2004.
-
- Todd AJ, Koerber HR. In: Wall and Melzack's Textbook of Pain. 5. McMahon SB, Koltzenburg M, editor. Philadelphia: Elsevier Churchill Livingston; 2006. Neuroanatomical substrates of spinal nociception; pp. 73–90.
-
- Brown AG. The dorsal horn of the spinal cord. Q J Exp Physiol. 1982;67:193–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

