Pin1 promotes transforming growth factor-beta-induced migration and invasion
- PMID: 19920136
- PMCID: PMC2804333
- DOI: 10.1074/jbc.M109.063826
Pin1 promotes transforming growth factor-beta-induced migration and invasion
Abstract
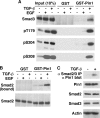
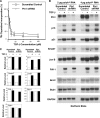
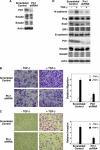
Transforming growth factor-beta (TGF-beta) regulates a wide variety of biological activities. It induces potent growth-inhibitory responses in normal cells but promotes migration and invasion of cancer cells. Smads mediate the TGF-beta responses. TGF-beta binding to the cell surface receptors leads to the phosphorylation of Smad2/3 in their C terminus as well as in the proline-rich linker region. The serine/threonine phosphorylation sites in the linker region are followed by the proline residue. Pin1, a peptidyl-prolyl cis/trans isomerase, recognizes phosphorylated serine/threonine-proline motifs. Here we show that Smad2/3 interacts with Pin1 in a TGF-beta-dependent manner. We further show that the phosphorylated threonine 179-proline motif in the Smad3 linker region is the major binding site for Pin1. Although epidermal growth factor also induces phosphorylation of threonine 179 and other residues in the Smad3 linker region the same as TGF-beta, Pin1 is unable to bind to the epidermal growth factor-stimulated Smad3. Further analysis suggests that phosphorylation of Smad3 in the C terminus is necessary for the interaction with Pin1. Depletion of Pin1 by small hairpin RNA does not significantly affect TGF-beta-induced growth-inhibitory responses and a number of TGF-beta/Smad target genes analyzed. In contrast, knockdown of Pin1 in human PC3 prostate cancer cells strongly inhibited TGF-beta-mediated migration and invasion. Accordingly, TGF-beta induction of N-cadherin, which plays an important role in migration and invasion, is markedly reduced when Pin1 is depleted in PC3 cells. Because Pin1 is overexpressed in many cancers, our findings highlight the importance of Pin1 in TGF-beta-induced migration and invasion of cancer cells.
Figures



References
-
- Roberts A. B., Sporn M. B. (1990) in Peptide Growth Factors and Their Receptors (Sporn M. B., Roberts A. B. eds) pp. 419–472, Springer-Verlag, Heidelberg, Germany
-
- Massagué J., Blain S. W., Lo R. S. (2000) Cell 103, 295–309 - PubMed
-
- Derynck R., Akhurst R. J., Balmain A. (2001) Nat. Genet. 29, 117–129 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

