A conserved aromatic lock for the tryptophan rotameric switch in TM-VI of seven-transmembrane receptors
- PMID: 19920139
- PMCID: PMC2823539
- DOI: 10.1074/jbc.M109.064725
A conserved aromatic lock for the tryptophan rotameric switch in TM-VI of seven-transmembrane receptors
Abstract
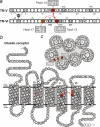
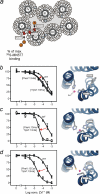
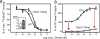
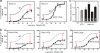
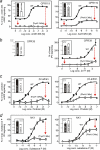
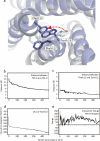
The conserved tryptophan in position 13 of TM-VI (Trp-VI:13 or Trp-6.48) of the CWXP motif located at the bottom of the main ligand-binding pocket in TM-VI is believed to function as a rotameric microswitch in the activation process of seven-transmembrane (7TM) receptors. Molecular dynamics simulations in rhodopsin demonstrated that rotation around the chi1 torsion angle of Trp-VI:13 brings its side chain close to the equally highly conserved Phe-V:13 (Phe-5.47) in TM-V. In the ghrelin receptor, engineering of high affinity metal-ion sites between these positions confirmed their close spatial proximity. Mutational analysis was performed in the ghrelin receptor with multiple substitutions and with Ala substitutions in GPR119, GPR39, and the beta(2)-adrenergic receptor as well as the NK1 receptor. In all of these cases, it was found that mutation of the Trp-VI:13 rotameric switch itself eliminated the constitutive signaling and strongly impaired agonist-induced signaling without affecting agonist affinity and potency. Ala substitution of Phe-V:13, the presumed interaction partner for Trp-VI:13, also in all cases impaired both the constitutive and the agonist-induced receptor signaling, but not to the same degree as observed in the constructs where Trp-VI:13 itself was mutated, but again without affecting agonist potency. In a proposed active receptor conformation generated by molecular simulations, where the extracellular segment of TM-VI is tilted inwards in the main ligand-binding pocket, Trp-VI:13 could rotate into a position where it obtained an ideal aromatic-aromatic interaction with Phe-V:13. It is concluded that Phe-V:13 can serve as an aromatic lock for the proposed active conformation of the Trp-VI:13 rotameric switch, being involved in the global movement of TM-V and TM-VI in 7TM receptor activation.
Figures
References
-
- Schwartz T. W., Rosenkilde M. M. (1996) Trends Pharmacol. Sci. 17, 213–216 - PubMed
-
- Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 481–519 - PubMed
-
- Pin J. P., Kniazeff J., Liu J., Binet V., Goudet C., Rondard P., Prézeau L. (2005) FEBS J. 272, 2947–2955 - PubMed
-
- Hubbell W. L., Altenbach C., Hubbell C. M., Khorana H. G. (2003) Adv. Protein Chem. 63, 243–290 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

