The amyloid precursor protein/protease nexin 2 Kunitz inhibitor domain is a highly specific substrate of mesotrypsin
- PMID: 19920152
- PMCID: PMC2804352
- DOI: 10.1074/jbc.M109.057216
The amyloid precursor protein/protease nexin 2 Kunitz inhibitor domain is a highly specific substrate of mesotrypsin
Abstract
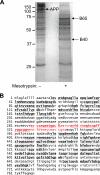
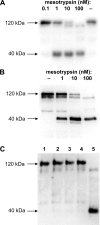
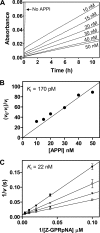
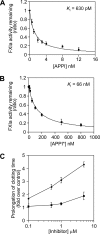
The amyloid precursor protein (APP) is a ubiquitously expressed transmembrane adhesion protein and the progenitor of amyloid-beta peptides. The major splice isoforms of APP expressed by most tissues contain a Kunitz protease inhibitor domain; secreted APP containing this domain is also known as protease nexin 2 and potently inhibits serine proteases, including trypsin and coagulation factors. The atypical human trypsin isoform mesotrypsin is resistant to inhibition by most protein protease inhibitors and cleaves some inhibitors at a substantially accelerated rate. Here, in a proteomic screen to identify potential physiological substrates of mesotrypsin, we find that APP/protease nexin 2 is selectively cleaved by mesotrypsin within the Kunitz protease inhibitor domain. In studies employing the recombinant Kunitz domain of APP (APPI), we show that mesotrypsin cleaves selectively at the Arg(15)-Ala(16) reactive site bond, with kinetic constants approaching those of other proteases toward highly specific protein substrates. Finally, we show that cleavage of APPI compromises its inhibition of other serine proteases, including cationic trypsin and factor XIa, by 2 orders of magnitude. Because APP/protease nexin 2 and mesotrypsin are coexpressed in a number of tissues, we suggest that processing by mesotrypsin may ablate the protease inhibitory function of APP/protease nexin 2 in vivo and may also modulate other activities of APP/protease nexin 2 that involve the Kunitz domain.
Figures



References
-
- Chen J. M., Ferec C. (2000) Pancreas 21, 57–62 - PubMed
-
- Ge X., Yamamoto S., Tsutsumi S., Midorikawa Y., Ihara S., Wang S. M., Aburatani H. (2005) Genomics 86, 127–141 - PubMed
-
- Yanai I., Benjamin H., Shmoish M., Chalifa-Caspi V., Shklar M., Ophir R., Bar-Even A., Horn-Saban S., Safran M., Domany E., Lancet D., Shmueli O. (2005) Bioinformatics 21, 650–659 - PubMed
-
- Diederichs S., Bulk E., Steffen B., Ji P., Tickenbrock L., Lang K., Zänker K. S., Metzger R., Schneider P. M., Gerke V., Thomas M., Berdel W. E., Serve H., Müller-Tidow C. (2004) Cancer Res. 64, 5564–5569 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

