N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death
- PMID: 19920154
- PMCID: PMC2807281
- DOI: 10.1074/jbc.M109.066191
N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death
Abstract
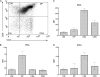
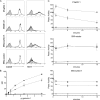
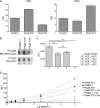
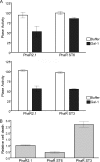
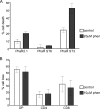
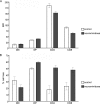
Galectin-1, a beta-galactoside-binding protein highly expressed in the thymus, induces apoptosis of specific thymocyte subsets and activated T cells. Galectin-1 binds to N- and O-glycans on several glycoprotein receptors, including CD7, CD43, and CD45. Here we show that galectin-1 signaling through CD45, which carries both N- and O-glycans, is regulated by CD45 isoform expression, core 2 O-glycan formation and the balance of N-glycan sialylation. Regulation of galectin-1 T cell death by O-glycans is mediated through CD45 phosphatase activity. While galectin-1 signaling in cells expressing low molecular weight isoforms of CD45 requires expression of core 2 O-glycans (high affinity ligands for galectin-1), galectin-1 signaling in cells expressing a high molecular weight isoform of CD45 does not require core 2 O-glycans, suggesting that a larger amount of core 1 O-glycans (low affinity ligands for galectin-1) is sufficient to overcome lack of core 2 O-glycans. Furthermore, regulation of galectin-1 signaling by alpha2,6-sialylation of N-glycans is not solely dependent on CD45 phosphatase activity and can be modulated by the relative expression of enzymes that attach sialic acid in an alpha2,6- or alpha2,3-linkage. Thus, N- and O-glycans modulate galectin-1 T cell death by distinct mechanisms, and different glycosylation events can render thymocytes susceptible or resistant to galectin-1.
Figures

References
-
- Hogquist K. A., Baldwin T. A., Jameson S. C. (2005) Nat. Rev. Immunol. 5, 772–782 - PubMed
-
- Liu S. D., Tomassian T., Bruhn K. W., Miller J. F., Poirier F., Miceli M. C. (2009) J. Immunol. 182, 5283–5295 - PubMed
-
- Perillo N. L., Pace K. E., Seilhamer J. J., Baum L. G. (1995) Nature 378, 736–739 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

