Transition state analogues in structures of ricin and saporin ribosome-inactivating proteins
- PMID: 19920175
- PMCID: PMC2787146
- DOI: 10.1073/pnas.0911606106
Transition state analogues in structures of ricin and saporin ribosome-inactivating proteins
Abstract
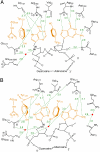
Ricin A-chain (RTA) and saporin-L1 (SAP) catalyze adenosine depurination of 28S rRNA to inhibit protein synthesis and cause cell death. We present the crystal structures of RTA and SAP in complex with transition state analogue inhibitors. These tight-binding inhibitors mimic the sarcin-ricin recognition loop of 28S rRNA and the dissociative ribocation transition state established for RTA catalysis. RTA and SAP share unique purine-binding geometry with quadruple pi-stacking interactions between adjacent adenine and guanine bases and 2 conserved tyrosines. An arginine at one end of the pi-stack provides cationic polarization and enhanced leaving group ability to the susceptible adenine. Common features of these ribosome-inactivating proteins include adenine leaving group activation, a remarkable lack of ribocation stabilization, and conserved glutamates as general bases for activation of the H(2)O nucleophile. Catalytic forces originate primarily from leaving group activation evident in both RTA and SAP in complex with transition state analogues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Transition state analogues rescue ribosomes from saporin-L1 ribosome inactivating protein.Biochemistry. 2009 Oct 20;48(41):9941-8. doi: 10.1021/bi901425h. Biochemistry. 2009. PMID: 19764816 Free PMC article.
-
Arginine residues on the opposite side of the active site stimulate the catalysis of ribosome depurination by ricin A chain by interacting with the P-protein stalk.J Biol Chem. 2013 Oct 18;288(42):30270-30284. doi: 10.1074/jbc.M113.510966. Epub 2013 Sep 3. J Biol Chem. 2013. PMID: 24003229 Free PMC article.
-
Pentameric organization of the ribosomal stalk accelerates recruitment of ricin a chain to the ribosome for depurination.J Biol Chem. 2010 Dec 31;285(53):41463-71. doi: 10.1074/jbc.M110.171793. Epub 2010 Oct 25. J Biol Chem. 2010. PMID: 20974854 Free PMC article.
-
Structures and Ribosomal Interaction of Ribosome-Inactivating Proteins.Molecules. 2016 Nov 21;21(11):1588. doi: 10.3390/molecules21111588. Molecules. 2016. PMID: 27879643 Free PMC article. Review.
-
Structures of eukaryotic ribosomal stalk proteins and its complex with trichosanthin, and their implications in recruiting ribosome-inactivating proteins to the ribosomes.Toxins (Basel). 2015 Feb 25;7(3):638-47. doi: 10.3390/toxins7030638. Toxins (Basel). 2015. PMID: 25723321 Free PMC article. Review.
Cited by
-
Pterin-based small molecule inhibitor capable of binding to the secondary pocket in the active site of ricin-toxin A chain.PLoS One. 2022 Dec 12;17(12):e0277770. doi: 10.1371/journal.pone.0277770. eCollection 2022. PLoS One. 2022. PMID: 36508422 Free PMC article.
-
Immunization with non-toxic variants of Shiga toxin type 2 (Stx2) generates high titers of protective antibodies.Dokl Biochem Biophys. 2015;460:23-5. doi: 10.1134/S160767291501007X. Epub 2015 Mar 13. Dokl Biochem Biophys. 2015. PMID: 25772984 No abstract available.
-
Soapwort Saporin L3 Expression in Yeast, Mutagenesis, and RNA Substrate Specificity.Biochemistry. 2015 Jul 28;54(29):4565-74. doi: 10.1021/acs.biochem.5b00405. Epub 2015 Jul 14. Biochemistry. 2015. PMID: 26091305 Free PMC article.
-
7-Substituted pterins provide a new direction for ricin A chain inhibitors.Eur J Med Chem. 2011 Sep;46(9):3608-15. doi: 10.1016/j.ejmech.2011.05.025. Epub 2011 May 20. Eur J Med Chem. 2011. PMID: 21641093 Free PMC article.
-
Conserved Arginines at the P-Protein Stalk Binding Site and the Active Site Are Critical for Ribosome Interactions of Shiga Toxins but Do Not Contribute to Differences in the Affinity of the A1 Subunits for the Ribosome.Infect Immun. 2016 Nov 18;84(12):3290-3301. doi: 10.1128/IAI.00630-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27600507 Free PMC article.
References
-
- Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987;262:5908–5912. - PubMed
-
- Amlot PL, et al. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood. 1993;82:2624–2633. - PubMed
-
- Messmann RA, et al. A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin Cancer Res. 2000;6:1302–1313. - PubMed
-
- Sausville EA, et al. Continuous infusion of the anti-CD22 immunotoxin IgG-RFB4-SMPT-dgA in patients with B-cell lymphoma: A phase I study. Blood. 1995;85:3457–3465. - PubMed
-
- Senderowicz AM, et al. Complete sustained response of a refractory, post-transplantation, large B-cell lymphoma to an anti-CD22 immunotoxin. Ann Intern Med. 1997;126:882–885. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

