Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression
- PMID: 19920183
- PMCID: PMC2795125
- DOI: 10.1158/0008-5472.CAN-09-1950
Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression
Abstract
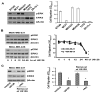
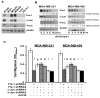
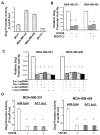
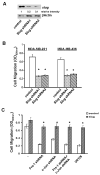
Cell migration is a critical step in cancer cell invasion. Recent studies have implicated the importance of the extracellular signal-regulated kinase (ERK) signaling pathway in cancer cell migration. However, the mechanism associated with ERK-regulated cell migration is poorly understood. Using a panel of breast cancer cell lines, we detected an excellent correlation between ERK activity and cell migration. Interestingly, we noticed that a 48-hour treatment with U0126 [specific mitogen-activated protein/ERK kinase (MEK)-1/2 inhibitor] was needed to significantly inhibit breast cancer cell migration, whereas this inhibitor blocked ERK activity within 1 hour. This observation suggests that ERK-dependent gene expression, rather than direct ERK signaling, is essential for cell migration. With further study, we found that ERK activity promoted the expression of the activator protein-1 (AP1) components Fra-1 and c-Jun, both of which were necessary for cell migration. Combination of U0126 treatment and Fra-1/c-Jun knockdown did not yield further reduction in cell migration than either alone, indicating that ERKs and Fra-1/c-Jun act by the same mechanism to facilitate cell migration. In an attempt to investigate the role of Fra-1/c-Jun in cell migration, we found that the ERK-Fra-1/c-Jun axis regulated slug expression in an AP1-dependent manner. Moreover, the occurrence of U0126-induced migratory inhibition coincided with slug reduction, and silencing slug expression abrogated breast cancer cell migration. These results suggest an association between ERK-regulated cell migration and slug expression. Indeed, cell migration was not significantly inhibited by U0126 treatment or Fra-1/c-Jun silencing in cells expressing slug transgene. Our study suggests that the ERK pathway regulates breast cancer cell migration by maintaining slug expression.
Figures

References
-
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature Rev Cancer. 2003;3:362–74. - PubMed
-
- Wehrle-Haller B, Imhof BA. Actin, microtubules and focal adhesion dynamics during cell migration. Int J Biochem Cell Biol. 2003;35:39–50. - PubMed
-
- Javelaud D, Laboureau J, Gabison E, Verrecchia F, Mauviel A. Disruption of basal JNK activity differentially affects key fibroblast functions important for wound healing. J Biol Chem. 2003;278:24624–8. - PubMed
-
- Meadows KN, Bryant P, Vincent PA, Pumiglia KM. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene. 2004;23:192–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

