Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas
- PMID: 19920199
- PMCID: PMC2789204
- DOI: 10.1158/0008-5472.CAN-08-3873
Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas
Abstract
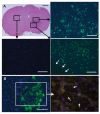
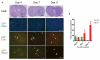
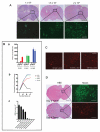
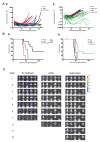
Delta24-RGD is an infectivity-augmented, conditionally replicative oncolytic adenovirus with significant antiglioma effects. Although intratumoral delivery of Delta24-RGD may be effective, intravascular delivery would improve successful application in humans. Due to their tumor tropic properties, we hypothesized that human mesenchymal stem cells (hMSC) could be harnessed as intravascular delivery vehicles of Delta24-RGD to human gliomas. To assess cellular events, green fluorescent protein-labeled hMSCs carrying Delta24-RGD (hMSC-Delta24) were injected into the carotid artery of mice harboring orthotopic U87MG or U251-V121 xenografts and brain sections were analyzed by immunofluorescence for green fluorescent protein and viral proteins (E1A and hexon) at increasing times. hMSC-Delta24 selectively localized to glioma xenografts and released Delta24-RGD, which subsequently infected glioma cells. To determine efficacy, mice were implanted with luciferase- labeled glioma xenografts, treated with hMSC-Delta24 or controls, and imaged weekly by bioluminescence imaging. Analysis of tumor size by bioluminescence imaging showed inhibition of glioma growth and eradication of tumors in hMSC-Delta24-treated animals compared with controls (P < 0.0001). There was an increase in median survival from 42 days in controls to 75.5 days in hMSC-Delta24-treated animals (P < 0.0001) and an increase in survival beyond 80 days from 0% to 37.5%, respectively. We conclude that intra-arterially delivered hMSC-Delta24 selectively localize to human gliomas and are capable of delivering and releasing Delta24-RGD into the tumor, resulting in improved survival and tumor eradication in subsets of mice.
Figures


Similar articles
-
Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma.Stem Cells. 2008 Mar;26(3):831-41. doi: 10.1634/stemcells.2007-0758. Epub 2008 Jan 10. Stem Cells. 2008. PMID: 18192232
-
Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas.Cancer Res. 2005 Apr 15;65(8):3307-18. doi: 10.1158/0008-5472.CAN-04-1874. Cancer Res. 2005. PMID: 15833864
-
The HDAC Inhibitors Scriptaid and LBH589 Combined with the Oncolytic Virus Delta24-RGD Exert Enhanced Anti-Tumor Efficacy in Patient-Derived Glioblastoma Cells.PLoS One. 2015 May 18;10(5):e0127058. doi: 10.1371/journal.pone.0127058. eCollection 2015. PLoS One. 2015. PMID: 25993039 Free PMC article.
-
Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas.Curr Gene Ther. 2009 Oct;9(5):422-7. doi: 10.2174/156652309789753356. Curr Gene Ther. 2009. PMID: 19860656 Free PMC article. Review.
-
Adenoviral virotherapy for malignant brain tumors.Expert Opin Biol Ther. 2009 Jun;9(6):737-47. doi: 10.1517/14712590902988451. Expert Opin Biol Ther. 2009. PMID: 19456208 Free PMC article. Review.
Cited by
-
Engineering antiphagocytic biomimetic drug carriers.Ther Deliv. 2013 Jul;4(7):825-39. doi: 10.4155/tde.13.54. Ther Deliv. 2013. PMID: 23883126 Free PMC article. Review.
-
[PVT1 Promoted the Proliferation and Migration Ability of BMSCs in Glioma C6 Microenvironment].Sichuan Da Xue Xue Bao Yi Xue Ban. 2021 May;52(3):438-444. doi: 10.12182/20210560203. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34018362 Free PMC article. Chinese.
-
Preclinical analysis of human mesenchymal stem cells: tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma.Oncotarget. 2019 Oct 22;10(58):6049-6061. doi: 10.18632/oncotarget.27071. eCollection 2019 Oct 22. Oncotarget. 2019. PMID: 31692882 Free PMC article.
-
Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma.J Neurosurg. 2021 Aug 27;136(3):757-767. doi: 10.3171/2021.3.JNS203045. Print 2022 Mar 1. J Neurosurg. 2021. PMID: 34450587 Free PMC article. Clinical Trial.
-
MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma.Aging (Albany NY). 2021 Feb 1;13(4):5055-5068. doi: 10.18632/aging.202424. Epub 2021 Feb 1. Aging (Albany NY). 2021. PMID: 33535172 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. - PubMed
-
- Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA-16672/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- CA-1094551/CA/NCI NIH HHS/United States
- R01 CA109451/CA/NCI NIH HHS/United States
- P50 CA116199/CA/NCI NIH HHS/United States
- P50 CA 127001/CA/NCI NIH HHS/United States
- CA-49639/CA/NCI NIH HHS/United States
- R01 CA115729/CA/NCI NIH HHS/United States
- P01 CA049639/CA/NCI NIH HHS/United States
- P50 CA127001/CA/NCI NIH HHS/United States
- CA-116199/CA/NCI NIH HHS/United States
- CA-55164/CA/NCI NIH HHS/United States
- P01 CA055164/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

