Regulation of macrophage motility by Irgm1
- PMID: 19920210
- PMCID: PMC2812558
- DOI: 10.1189/jlb.0509299
Regulation of macrophage motility by Irgm1
Abstract
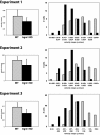
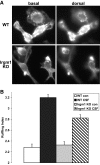
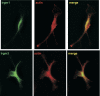
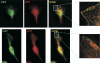
IRG are a family of IFN-regulated proteins that are critical for resistance to infection. Mouse IRG proteins are divided into GMS and GKS subfamilies, based on a sequence within the G1 GTP-binding motif. The GMS proteins have a particularly profound impact on immunity, as typified by Irgm1, of which absence leads to a complete loss of resistance to a variety of intracellular bacteria and protozoa. The underlying molecular and cellular mechanisms are not clear. Here, we use time-lapse microscopy and cell-tracking analysis to demonstrate that Irgm1 is required for motility of IFN-gamma-activated macrophages. The absence of Irgm1 led to decreased actin remodeling at the leading edge of migrating macrophages, as well as decreased Rac activation. Although Irgm1 did not localize to the leading edge of migrating macrophages, it was found to regulate the localization of a GKS IRG protein, Irgb6, which in turn, concentrated on the plasma membrane in the advancing lamellipodia, in close apposition to molecular components that regulate membrane remodeling, including Rac, paxillin, and actin. Thus, Irgm1 likely controls macrophage motility by regulating the positioning of specific GKS IRG proteins to the plasma membrane, which in turn, modulate cytoskeletal remodeling and membrane dynamics.
Figures


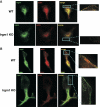

Similar articles
-
Loss of the interferon-γ-inducible regulatory immunity-related GTPase (IRG), Irgm1, causes activation of effector IRG proteins on lysosomes, damaging lysosomal function and predicting the dramatic susceptibility of Irgm1-deficient mice to infection.BMC Biol. 2016 Apr 20;14:33. doi: 10.1186/s12915-016-0255-4. BMC Biol. 2016. PMID: 27098192 Free PMC article.
-
Impaired macrophage function underscores susceptibility to Salmonella in mice lacking Irgm1 (LRG-47).J Immunol. 2007 Nov 15;179(10):6963-72. doi: 10.4049/jimmunol.179.10.6963. J Immunol. 2007. PMID: 17982087
-
Balance of Irgm protein activities determines IFN-gamma-induced host defense.J Leukoc Biol. 2009 May;85(5):877-85. doi: 10.1189/jlb.1008599. Epub 2009 Jan 27. J Leukoc Biol. 2009. PMID: 19176402 Free PMC article.
-
Cell migration: GAPs between membrane traffic and the cytoskeleton.EMBO Rep. 2001 Apr;2(4):277-81. doi: 10.1093/embo-reports/kve072. EMBO Rep. 2001. PMID: 11306546 Free PMC article. Review.
-
Filling gaps in signaling to actin cytoskeletal remodeling.Dev Cell. 2003 Apr;4(4):444-5. doi: 10.1016/s1534-5807(03)00098-4. Dev Cell. 2003. PMID: 12689583 Review.
Cited by
-
How did we get here? Insights into mechanisms of immunity-related GTPase targeting to intracellular pathogens.Curr Opin Microbiol. 2022 Oct;69:102189. doi: 10.1016/j.mib.2022.102189. Epub 2022 Aug 11. Curr Opin Microbiol. 2022. PMID: 35963099 Free PMC article. Review.
-
Metabolic Alterations Contribute to Enhanced Inflammatory Cytokine Production in Irgm1-deficient Macrophages.J Biol Chem. 2017 Mar 17;292(11):4651-4662. doi: 10.1074/jbc.M116.770735. Epub 2017 Feb 1. J Biol Chem. 2017. PMID: 28154172 Free PMC article.
-
IRGM1 regulates oxidized LDL uptake by macrophage via actin-dependent receptor internalization during atherosclerosis.Sci Rep. 2013;3:1867. doi: 10.1038/srep01867. Sci Rep. 2013. PMID: 23689639 Free PMC article.
-
Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole.Cell Microbiol. 2010 Jul;12(7):939-61. doi: 10.1111/j.1462-5822.2010.01443.x. Epub 2010 Jan 26. Cell Microbiol. 2010. PMID: 20109161 Free PMC article.
-
Factors regulated by interferon gamma and hypoxia-inducible factor 1A contribute to responses that protect mice from Coccidioides immitis infection.BMC Microbiol. 2012 Sep 24;12:218. doi: 10.1186/1471-2180-12-218. BMC Microbiol. 2012. PMID: 23006927 Free PMC article.
References
-
- Taylor G A. IRG proteins: key mediators of interferon-regulated host resistance to intracellular pathogens. Cell Microbiol. 2007;9:1099–1107. - PubMed
-
- Martens S, Howard J. The interferon-inducible GTPases. Annu Rev Cell Dev Biol. 2006;22:559–589. - PubMed
-
- Taylor G A, Feng C G, Sher A. Control of IFN-γ-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

