Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle
- PMID: 19920217
- PMCID: PMC2822482
- DOI: 10.1152/ajpendo.00375.2009
Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle
Abstract
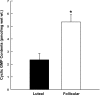
The follicular phase of the ovine ovarian cycle demonstrates parallel increases in ovarian estrogens and uterine blood flow (UBF). Although estrogen and nitric oxide contribute to the rise in UBF, the signaling pathway remains unclear. We examined the relationship between the rise in UBF during the ovarian cycle of nonpregnant sheep and changes in the uterine vascular cGMP-dependent pathway and large-conductance Ca(2+)-activated K(+) channels (BK(Ca)). Nonpregnant ewes (n = 19) were synchronized to either follicular or luteal phase using a vaginal progesterone-releasing device (CIDR), followed by intramuscular PGF(2alpha), CIDR removal, and treatment with pregnant mare serum gonadotropin. UBF was measured with flow probes before tissue collection, and second-generation uterine artery segments were collected from nine follicular and seven luteal phase ewes. The pore-forming alpha- and regulatory beta-subunits that constitute the BK(Ca), soluble guanylyl cyclase (sGC), and cGMP-dependent protein kinase G (cPKG) isoforms (cPKG(1alpha) and cPKG(1beta)) were measured by Western analysis and cGMP levels by RIA. BK(Ca) subunits were localized by immunohistochemistry. UBF rose >3-fold (P < 0.04) in follicular phase ewes, paralleling a 2.3-fold rise in smooth muscle cGMP and 32% increase in cPKG(1alpha) (P < 0.05). sGC, cPKG(1beta), and the BK(Ca) alpha-subunit were unchanged. Notably, expression of beta(1)- and beta(2)-regulatory subunits rose 51 and 79% (P <or= 0.05), respectively. Increases in endogenous ovarian estrogens in follicular-phase ewes result in increases in UBF associated with upregulation of the cGMP- and cPKG-dependent pathway and increased vascular BK(Ca) beta/alpha-subunit stoichiometry, suggesting enhanced BK(Ca) activation contributes to the follicular phase rise in UBF.
Figures





Similar articles
-
Large conductance Ca2+-activated and voltage-activated K+ channels contribute to the rise and maintenance of estrogen-induced uterine vasodilation and maintenance of blood pressure.Endocrinology. 2012 Dec;153(12):6012-20. doi: 10.1210/en.2012-1717. Epub 2012 Oct 15. Endocrinology. 2012. PMID: 23070547 Free PMC article.
-
Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle.Am J Physiol Heart Circ Physiol. 2009 Jun;296(6):H1878-87. doi: 10.1152/ajpheart.01185.2008. Am J Physiol Heart Circ Physiol. 2009. PMID: 19470517 Free PMC article.
-
Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy.J Soc Gynecol Investig. 2005 Sep;12(6):402-8. doi: 10.1016/j.jsgi.2005.04.009. J Soc Gynecol Investig. 2005. PMID: 15979352
-
Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows.J Anim Sci. 1982;55 Suppl 2:32-42. J Anim Sci. 1982. PMID: 6765316 Review.
-
Review article: steroid hormones and uterine vascular adaptation to pregnancy.Reprod Sci. 2008 Apr;15(4):336-48. doi: 10.1177/1933719108317975. Reprod Sci. 2008. PMID: 18497342 Free PMC article. Review.
Cited by
-
Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries.Hypertension. 2011 Dec;58(6):1132-9. doi: 10.1161/HYPERTENSIONAHA.111.179952. Epub 2011 Oct 31. Hypertension. 2011. PMID: 22042813 Free PMC article.
-
Ca2+-Activated K+ Channels and the Regulation of the Uteroplacental Circulation.Int J Mol Sci. 2023 Jan 10;24(2):1349. doi: 10.3390/ijms24021349. Int J Mol Sci. 2023. PMID: 36674858 Free PMC article. Review.
-
Large conductance Ca2+-activated and voltage-activated K+ channels contribute to the rise and maintenance of estrogen-induced uterine vasodilation and maintenance of blood pressure.Endocrinology. 2012 Dec;153(12):6012-20. doi: 10.1210/en.2012-1717. Epub 2012 Oct 15. Endocrinology. 2012. PMID: 23070547 Free PMC article.
-
Effect of Oxidative Stress on the Estrogen-NOS-NO-KCa Channel Pathway in Uteroplacental Dysfunction: Its Implication in Pregnancy Complications.Oxid Med Cell Longev. 2019 Feb 10;2019:9194269. doi: 10.1155/2019/9194269. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30881600 Free PMC article. Review.
-
VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy.Am J Pathol. 2013 Nov;183(5):1596-1607. doi: 10.1016/j.ajpath.2013.07.031. Epub 2013 Sep 13. Am J Pathol. 2013. PMID: 24036251 Free PMC article.
References
-
- Carnegie JA, Robertson HA. Conjugated and unconjugated estrogens in fetal and maternal fluids of the pregnant ewe: a possible role for estrone sulfate during early pregnancy. Biol Reprod 19: 202–211, 1978. - PubMed
-
- Chang S, Hypolite JA, Velez M, Changolkar A, Wein AJ, Chacko S, DiSanto ME. Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am J Physiol Regul Integr Comp Physiol 287: R950–R960, 2004. - PubMed
-
- Chen L, Shipston MJ. Cloning of potassium channel splice variants from tissues and cells. Methods Mol Biol 491: 35–60, 2009. - PubMed
-
- Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol Heart Circ Physiol 272: H2765–H2773, 1997. - PubMed
-
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

