Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat
- PMID: 19920218
- PMCID: PMC2822484
- DOI: 10.1152/ajpendo.00437.2009
Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat
Abstract
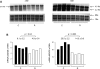
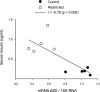
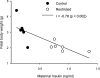
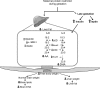
Suboptimal developmental environments program offspring to lifelong metabolic problems. The aim of this study was to determine the impact of protein restriction in pregnancy on maternal liver lipid metabolism at 19 days of gestation (dG) and its effect on fetal brain development. Control (C) and restricted (R) mothers were fed with isocaloric diets containing 20 and 10% of casein. At 19 dG, maternal blood and livers and fetal livers and brains were collected. Serum insulin and leptin levels were determinate in mothers. Maternal and fetal liver lipid and fetal brain lipid quantification were performed. Maternal liver and fetal brain fatty acids were quantified by gas chromatography. In mothers, liver desaturase and elongase mRNAs were measured by RT-PCR. Maternal body and liver weights were similar in both groups. However, fat body composition, including liver lipids, was lower in R mothers. A higher fasting insulin at 19 dG in the R group was observed (C = 0.2 +/- 0.04 vs. R = 0.9 +/- 0.16 ng/ml, P < 0.01) and was inversely related to early growth retardation. Serum leptin in R mothers was significantly higher than that observed in C rats (C = 5 +/- 0.1 vs. R = 7 +/- 0.7 ng/ml, P < 0.05). In addition, protein restriction significantly reduced gene expression in maternal liver of desaturases and elongases and the concentration of arachidonic (AA) and docosahexanoic (DHA) acids. In fetus from R mothers, a low body weight (C = 3 +/- 0.3 vs. R = 2 +/- 0.1 g, P < 0.05), as well as liver and brain lipids, including the content of DHA in the brain, was reduced. This study showed that protein restriction during pregnancy may negatively impact normal fetal brain development by changes in maternal lipid metabolism.
Figures
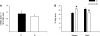
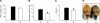
Similar articles
-
Effect of reduced dietary protein intake on hepatic and plasma essential fatty acid concentrations in the adult female rat: effect of pregnancy and consequences for accumulation of arachidonic and docosahexaenoic acids in fetal liver and brain.Br J Nutr. 2002 Oct;88(4):379-87. doi: 10.1079/BJN2002664. Br J Nutr. 2002. PMID: 12323087
-
Changes in milk composition in obese rats consuming a high-fat diet.Br J Nutr. 2016 Feb 14;115(3):538-46. doi: 10.1017/S0007114515004547. Epub 2015 Nov 26. Br J Nutr. 2016. PMID: 26608475
-
Protein restriction in the rat negatively impacts long-chain polyunsaturated fatty acid composition and mammary gland development at the end of gestation.Arch Med Res. 2013 Aug;44(6):429-36. doi: 10.1016/j.arcmed.2013.08.002. Epub 2013 Sep 17. Arch Med Res. 2013. PMID: 24051037
-
Maternal and fetal brain contents of docosahexaenoic acid (DHA) and arachidonic acid (AA) at various essential fatty acid (EFA), DHA and AA dietary intakes during pregnancy in mice.Prostaglandins Leukot Essent Fatty Acids. 2008 Mar;78(3):159-69. doi: 10.1016/j.plefa.2008.01.004. Epub 2008 Mar 14. Prostaglandins Leukot Essent Fatty Acids. 2008. PMID: 18343099 Review.
-
The ins and outs of maternal-fetal fatty acid metabolism.Acta Biochim Pol. 2015;62(3):499-507. doi: 10.18388/abp.2015_1067. Epub 2015 Sep 8. Acta Biochim Pol. 2015. PMID: 26345097 Review.
Cited by
-
Macronutrient Intake in Pregnancy and Child Cognitive and Behavioural Outcomes.Children (Basel). 2021 May 20;8(5):425. doi: 10.3390/children8050425. Children (Basel). 2021. PMID: 34065501 Free PMC article.
-
Maternal supplementation of α-linolenic acid in normal and protein-restricted diets modulate lipid metabolism, adipose tissue growth and leptin levels in the suckling offspring.Eur J Nutr. 2015 Aug;54(5):761-70. doi: 10.1007/s00394-014-0755-3. Epub 2014 Aug 11. Eur J Nutr. 2015. PMID: 25109672
-
Gestational protein restriction induces alterations in placental morphology and mitochondrial function in rats during late pregnancy.J Mol Histol. 2013 Dec;44(6):629-37. doi: 10.1007/s10735-013-9522-7. Epub 2013 Jul 25. J Mol Histol. 2013. PMID: 23884563
-
Deletion of pleiotrophin impairs glucose tolerance and liver metabolism in pregnant mice: Moonlighting role of glycerol kinase.FASEB J. 2021 Oct;35(10):e21911. doi: 10.1096/fj.202101181R. FASEB J. 2021. PMID: 34551152 Free PMC article.
-
Hepatocyte ballooning and steatosis in early and late gestation without liver malfunction: Effects of low protein/high carbohydrate diet.PLoS One. 2024 Jan 2;19(1):e0294062. doi: 10.1371/journal.pone.0294062. eCollection 2024. PLoS One. 2024. PMID: 38166013 Free PMC article.
References
-
- AOAC (Association of Official Analytical Chemists) Official Methods of Analysis of AOAC International ( 17th ed.), edited by Harwitz W. Gaithersburg, MD: AOAC International, 2002, p. 920.05–920.39.
-
- Bautista CJ, Boeck L, Larrea F, Nathanielsz PW, Zambrano E. Effects of a maternal low protein isocaloric diet on milk leptin and progeny serum leptin concentration and appetitive behavior in the first 21 days of neonatal life in the rat. Pediatr Res 63: 358–363, 2008 - PubMed
-
- Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging 8: 163–174, 2004 - PubMed
-
- Burdge GC, Dunn RL, Wootton SA, Jackson AA. Effect of reduced dietary protein intake on hepatic and plasma essential fatty acid concentrations in the adult female rat: effect of pregnancy and consequences for accumulation of arachidonic and docosahexaenoic acids in fetal liver and brain. Br J Nutr 88: 379–387, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

