Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms
- PMID: 19920274
- PMCID: PMC3515682
- DOI: 10.7326/0003-4819-151-10-200911170-00010
Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms
Erratum in
- Ann Intern Med. 2010 Jan 19;152(2):136
Abstract
Background: Despite trials of mammography and widespread use, optimal screening policy is controversial.
Objective: To evaluate U.S. breast cancer screening strategies.
Design: 6 models using common data elements.
Data sources: National data on age-specific incidence, competing mortality, mammography characteristics, and treatment effects.
Target population: A contemporary population cohort.
Time horizon: Lifetime.
Perspective: Societal.
Interventions: 20 screening strategies with varying initiation and cessation ages applied annually or biennially.
Outcome measures: Number of mammograms, reduction in deaths from breast cancer or life-years gained (vs. no screening), false-positive results, unnecessary biopsies, and overdiagnosis.
Results of base-case analysis: The 6 models produced consistent rankings of screening strategies. Screening biennially maintained an average of 81% (range across strategies and models, 67% to 99%) of the benefit of annual screening with almost half the number of false-positive results. Screening biennially from ages 50 to 69 years achieved a median 16.5% (range, 15% to 23%) reduction in breast cancer deaths versus no screening. Initiating biennial screening at age 40 years (vs. 50 years) reduced mortality by an additional 3% (range, 1% to 6%), consumed more resources, and yielded more false-positive results. Biennial screening after age 69 years yielded some additional mortality reduction in all models, but overdiagnosis increased most substantially at older ages.
Results of sensitivity analysis: Varying test sensitivity or treatment patterns did not change conclusions.
Limitation: Results do not include morbidity from false-positive results, patient knowledge of earlier diagnosis, or unnecessary treatment.
Conclusion: Biennial screening achieves most of the benefit of annual screening with less harm. Decisions about the best strategy depend on program and individual objectives and the weight placed on benefits, harms, and resource considerations.
Primary funding source: National Cancer Institute.
Figures
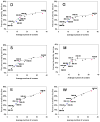
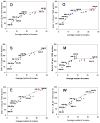
References
-
- Jemal A, Siegel R, Ward Em Hao Y, Xu J, thus MJ. Cancer Statistics 2009. American Cancer Society CA Cancer. J Clin. 2009;59:225–249. - PubMed
-
- Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359:909–19. - PubMed
-
- Tabar L, Vitak B, Chen H, Duffy S, Yen MF, Chiang CG, Krusemo UB, Tot T, Smith RA. The Swedish Two-County trial twenty years later: Updated mortality results and new insights from long-term follow-up. Radiologic Clinics of North America. 2000;38 (4):625–51. - PubMed
-
- International Agency for Research on Cancer. Breast Cancer Screening. In: Vainio H, Bianchini F, editors. IARC Handbook on Cancer Prevention, Report No 7. Lyon: IARC; 2002.
-
- Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial. Lancet. 2006;368:2053–60. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA063740/CA/NCI NIH HHS/United States
- U01CA69976/CA/NCI NIH HHS/United States
- U01 CA070040/CA/NCI NIH HHS/United States
- 2U01CA088248/CA/NCI NIH HHS/United States
- U01CA70013/CA/NCI NIH HHS/United States
- U01 CA086082/CA/NCI NIH HHS/United States
- 2U01CA088283/CA/NCI NIH HHS/United States
- 2U01CA088270/CA/NCI NIH HHS/United States
- U01CA63736/CA/NCI NIH HHS/United States
- U01CA86082/CA/NCI NIH HHS/United States
- U01 CA088248/CA/NCI NIH HHS/United States
- F32 CA125984/CA/NCI NIH HHS/United States
- U01CA70040/CA/NCI NIH HHS/United States
- U01CA63740/CA/NCI NIH HHS/United States
- U01 CA063731/CA/NCI NIH HHS/United States
- U01 CA086076/CA/NCI NIH HHS/United States
- HHSN261200800002C/CA/NCI NIH HHS/United States
- HHSN261200800769P/PHS HHS/United States
- U01 CA069976/CA/NCI NIH HHS/United States
- U01CA86076/CA/NCI NIH HHS/United States
- U01 CA063736/CA/NCI NIH HHS/United States
- U01 CA070013/CA/NCI NIH HHS/United States
- U01CA63731/CA/NCI NIH HHS/United States
- U01 CA088270/CA/NCI NIH HHS/United States
- U01 CA088283/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
