Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks
- PMID: 19920352
- PMCID: PMC2786795
- DOI: 10.1172/JCI39401
Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks
Abstract
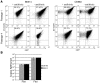
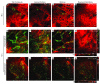
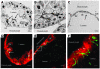
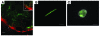
Lyme disease is caused by transmission of the spirochete Borrelia burgdorferi from ticks to humans. Although much is known about B. burgdorferi replication, the routes and mechanisms by which it disseminates within the tick remain unclear. To better understand this process, we imaged live, infectious B. burgdorferi expressing a stably integrated, constitutively expressed GFP reporter. Using isolated tick midguts and salivary glands, we observed B. burgdorferi progress through the feeding tick via what we believe to be a novel, biphasic mode of dissemination. In the first phase, replicating spirochetes, positioned at varying depths throughout the midgut at the onset of feeding, formed networks of nonmotile organisms that advanced toward the basolateral surface of the epithelium while adhering to differentiating, hypertrophying, and detaching epithelial cells. In the second phase of dissemination, the nonmotile spirochetes transitioned into motile organisms that penetrated the basement membrane and entered the hemocoel, then migrated to and entered the salivary glands. We designated the first phase of dissemination "adherence-mediated migration" and provided evidence that it involves the inhibition of spirochete motility by one or more diffusible factors elaborated by the feeding tick midgut. Our studies, which we believe are the first to relate the transmission dynamics of spirochetes to the complex morphological and developmental changes that the midgut and salivary glands undergo during engorgement, challenge the conventional viewpoint that dissemination of Lyme disease-causing spirochetes within ticks is exclusively motility driven.
Figures



Similar articles
-
Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors.J Clin Microbiol. 2001 Nov;39(11):4145-8. doi: 10.1128/JCM.39.11.4145-4148.2001. J Clin Microbiol. 2001. PMID: 11682544 Free PMC article.
-
Passage through Ixodes scapularis ticks enhances the virulence of a weakly pathogenic isolate of Borrelia burgdorferi.Infect Immun. 2010 Jan;78(1):138-44. doi: 10.1128/IAI.00470-09. Epub 2009 Oct 12. Infect Immun. 2010. PMID: 19822652 Free PMC article.
-
OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands.J Clin Invest. 2004 Jan;113(2):220-30. doi: 10.1172/JCI19894. J Clin Invest. 2004. PMID: 14722614 Free PMC article.
-
Physiologic and Genetic Factors Influencing the Zoonotic Cycle of Borrelia burgdorferi.Curr Top Microbiol Immunol. 2018;415:63-82. doi: 10.1007/82_2017_43. Curr Top Microbiol Immunol. 2018. PMID: 28864829 Review.
-
Pathophysiology of the Lyme disease spirochete, Borrelia burgdorferi, in ixodid ticks.Rev Infect Dis. 1989 Sep-Oct;11 Suppl 6:S1442-50. doi: 10.1093/clinids/11.supplement_6.s1442. Rev Infect Dis. 1989. PMID: 2682956 Review.
Cited by
-
Prevention of tick-borne diseases: challenge to recent medicine.Biologia (Bratisl). 2022;77(6):1533-1554. doi: 10.1007/s11756-021-00966-9. Epub 2022 Mar 9. Biologia (Bratisl). 2022. PMID: 35283489 Free PMC article. Review.
-
Correlative cryo-fluorescence and cryo-scanning electron microscopy as a straightforward tool to study host-pathogen interactions.Sci Rep. 2015 Dec 10;5:18029. doi: 10.1038/srep18029. Sci Rep. 2015. PMID: 26658551 Free PMC article.
-
Assessment of MALDI-TOF MS biotyping for Borrelia burgdorferi sl detection in Ixodes ricinus.PLoS One. 2017 Sep 26;12(9):e0185430. doi: 10.1371/journal.pone.0185430. eCollection 2017. PLoS One. 2017. PMID: 28950023 Free PMC article.
-
Identification of Borrelia protein candidates in mouse skin for potential diagnosis of disseminated Lyme borreliosis.Sci Rep. 2017 Dec 1;7(1):16719. doi: 10.1038/s41598-017-16749-9. Sci Rep. 2017. PMID: 29196626 Free PMC article.
-
Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules.Mol Microbiol. 2012 Dec;86(5):1116-31. doi: 10.1111/mmi.12045. Epub 2012 Oct 24. Mol Microbiol. 2012. PMID: 23095033 Free PMC article.
References
-
- WHO. 2008. World health statistics. http://www.who.int/whosis/whostat/2008/en/index.html.

