A PI3 kinase inhibitor found to activate bestrophin 3
- PMID: 19920768
- PMCID: PMC3052421
- DOI: 10.1097/FJC.0b013e3181c87c85
A PI3 kinase inhibitor found to activate bestrophin 3
Abstract
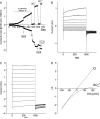
Bestrophin 3 (Best3), a member of bestrophin Cl channel family, is a CaCl(cGMP) channel candidate in vascular smooth muscle cells. The mechanism for its activation remains unclear. In previous studies, we reported that an autoinhibitory domain ((356)IPSFLGS(362)) existed in Best3 C-terminus and when the autoinhibitory domain was mutated, the Best3 channel was dramatically activated. In this study, we further dissected the roles of the C-terminal sequence in Best3 activation. We found that there were eight basic amino acids downstream of the AI domain within the region (384-397), which were also involved in Best3 activation. Mutations of these basic amino acids significantly activated Best3 as a Cl channel. Led by the assumption that the basic amino acids may be involved in the Best3 C-terminal membrane association through binding to membranous phospholipids, we discovered that PI3Kalpha inhibitor IV could strongly activate Best3.
Figures



References
-
- Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–247. - PubMed
-
- Marquardt A, Stohr H, Passmore LA, et al. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum Mol Genet. 1998;7:1517–1525. - PubMed
-
- Hartzell C, Qu Z, Putzier I, et al. Looking chloride channels straight in the eye: bestrophins, lipofuscinosis, and retinal degeneration. Physiology. 2005;20:292–302. - PubMed
-
- Hartzell HC, Qu Z, Yu K, et al. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

