Kidney after nonrenal transplantation-the impact of alemtuzumab induction
- PMID: 19920779
- PMCID: PMC3032633
- DOI: 10.1097/TP.0b013e3181b4aaf5
Kidney after nonrenal transplantation-the impact of alemtuzumab induction
Abstract
Background: Calcineurin inhibitor nephrotoxicity in nonrenal allograft recipients can lead to end-stage renal disease and the need for kidney transplantation. We sought to evaluate the role of alemtuzumab induction in this population.
Patients and methods: We evaluated 144 patients undergoing kidney transplantation after nonrenal transplantation between May 18, 1998, and October 8, 2007. Seventy-two patients transplanted between January 15, 2003, and October 8, 2007, received alemtuzumab induction and continued their pretransplant immunosuppression. Seventy-two patients transplanted between May 18, 1998, and July 21, 2007, did not receive alemtuzumab induction, but received additional steroids and maintenance immunosuppression. Donor and recipient demographics were comparable.
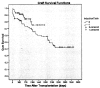
Results: Overall, 1- and 3-year patient survival and renal function were comparable between the two groups. One- and 3-year graft survival was 93.0% and 75.3% in the alemtuzumab group and 83.3% and 68.7% in the no alemtuzumab group, respectively (P=0.051). The incidence of acute rejection was lower in the alemtuzumab group, 15.3%, than in the no alemtuzumab group, 41.7% (P=0.0001). The incidence of delayed graft function was lower in the alemtuzumab group, 9.7%, than in the no alemtuzumab group, 25.0% (P=0.003). The incidence of viral complications was comparable.
Conclusion: Alemtuzumab induction with simple resumption of baseline immunosuppression in patients undergoing kidney transplantation after nonrenal transplantation represents a reasonable immunosuppressive strategy.
Figures
References
-
- Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:331. - PubMed
-
- Waldmann H. A personal history of the CAMPATH-1H antibody. Med Oncol. 2002;19(suppl):S3. - PubMed
-
- Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative Campath lH, and low- dose cyclosporine monotherapy in renal allograft recipients. Lancet. 1998;351:1701. - PubMed
-
- Stuart FP, Leventhal JR, Kaufman DB. Alemtuzumab facilitates prednisone free immunosuppression in kidney transplant recipients with no early rejection. Am J Transplant. 2002;2(suppl):397. - PubMed
-
- Knechtle SJ, Pirsch JD, Fechner HJ, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation results of a pilot study. Am J Transplant. 2003;3:722. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources