Pharmacotherapy of HIV-1 Infection: Focus on CCR5 Antagonist Maraviroc
- PMID: 19920876
- PMCID: PMC2777720
- DOI: 10.4137/cmt.s2365
Pharmacotherapy of HIV-1 Infection: Focus on CCR5 Antagonist Maraviroc
Abstract
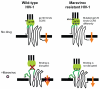
Sustained inhibition of HIV-1, the goal of antiretroviral therapy, is often impeded by the emergence of viral drug resistance. For patients infected with HIV-1 resistant to conventional drugs from the viral reverse transcriptase and protease inhibitor classes, the recently approved entry and integration inhibitors effectively suppress HIV-1 and offer additional therapeutic options. Entry inhibitors are particularly attractive because, unlike conventional antiretrovirals, they target HIV-1 extracellularly, thereby sparing cells from both viral- and drug-induced toxicities. The fusion inhibitor enfuvirtide and the CCR5 antagonist maraviroc are the first entry inhibitors licensed for patients with drug-resistant HIV-1, with maraviroc restricted to those infected with CCR5-tropic HIV-1 (R5 HIV-1) only. Vicriviroc (another CCR5 antagonist) is in Phase III clinical trials, whereas the CCR5 antibodies PRO 140 and HGS 004 are in early stages of clinical development. Potent antiviral synergy between maraviroc and CCR5 antibodies, coupled with distinct patterns of resistance, suggest their combinations might be particularly effective in patients. In addition, given that oral administration of maraviroc achieves high drug levels in cervicovaginal fluid, combinations of maraviroc and other CCR5 inhibitors could be effective in preventing HIV-1 transmission. Moreover, since CCR5 antagonists prevent rejection of transplanted organs, maraviroc could both suppress HIV-1 and prolong organ survival for the growing number of HIV-1 patients with kidney or liver failure necessitating organ transplantation. Thus, maraviroc offers an important treatment option for patients with drug-resistant R5 HIV-1, who presently account for >50% of drug-resistance cases.
Figures


References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–60. - PubMed
-
- Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14(3):309–18. - PubMed
-
- Yerly S, Vora S, Rizzardi P, et al. Acute HIV infection: impact on the spread of HIV and transmission of drug resistance. Aids. 2001;15(17):2287–92. - PubMed
-
- Kuhmann SE, Hartley O. Targeting chemokine receptors in HIV: a status report. Annu Rev Pharmacol Toxicol. 2008;48:425–61. - PubMed
-
- Robertson D. US FDA approves new class of HIV therapeutics. Nat Biotechnol. 2003;21(5):470–1. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
