Discovery, development, and clinical application of sugammadex sodium, a selective relaxant binding agent
- PMID: 19920893
- PMCID: PMC2761174
- DOI: 10.2147/dddt.s2757
Discovery, development, and clinical application of sugammadex sodium, a selective relaxant binding agent
Abstract
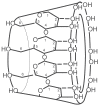
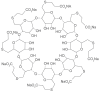
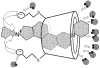
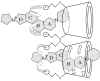
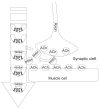
Neuromuscular blockade, induced by neuromuscular blocking agents, has allowed prescribed immobility, improved surgical exposure, optimal airway management conditions, and facilitated mechanical ventilation. However, termination of the effects of neuromuscular blocking agents has, until now, remained limited. A novel cyclodextrin encapsulation process offers improved termination of the paralytic effects of aminosteroidal non-depolarizing neuromuscular blocking agents. Sugammadex sodium is the first in a new class of drug called selective relaxant binding agents. Currently, in clinical trials, sugammadex, a modified gamma cyclodextrin, has shown consistent and rapid termination of neuromuscular blockade with few side effects. The pharmacology of cyclodextrins in general and sugammadex in particular, together with the results of current clinical research are reviewed. The ability of sugammadex to terminate the action of neuromuscular blocking agents by direct encapsulation is compared to the indirect competitive antagonism of their effects by cholinesterase inhibitors. Also discussed are the clinical implications that extend beyond fast, effective reversal, including numerous potential perioperative benefits.
Keywords: encapsulation; modified cyclodextrin; muscle relaxants; neuromuscular blockade reversal; selective relaxant binding agent (SRBA); sugammadex.
Figures
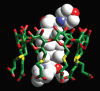
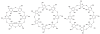
References
-
- Adam JM, Bennett DJ, Bom A, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J Med Chem. 2002;45:1806–16. - PubMed
-
- Alvarez-Gomez JA, Wattwil M, Vanacker B, et al. Reversal of vecuronium-induced shallow neuromuscular blockade is significantly faster with sugammadex compared with neostigmine. Eur J Anaesthesiol. 2007;24(Suppl 39):124–5.
-
- Amao R, Zornow MH, Cowan MR, et al. Sugammadex safely reverses rocuronium-induced blockade in patients with pulmonary disease. Anesthesiology. 2007;107:A1582.
-
- Appelboam R, Mulder R, Saddler J. Atracurium associated with post operative residual curarization. Correspondence. Br J Anaesthesiol. 2003;90:523–8. - PubMed
-
- Baillard C, Gehan G, Reboul-Marty J, et al. Residual curarization in the recovery room after vecuronium. Br J of Anaesthesiol. 2000;84:394–5. - PubMed
LinkOut - more resources
Full Text Sources
Medical

