Pharmacological control of neutrophil-mediated inflammation: strategies targeting calcium handling by activated polymorphonuclear leukocytes
- PMID: 19920897
- PMCID: PMC2761182
Pharmacological control of neutrophil-mediated inflammation: strategies targeting calcium handling by activated polymorphonuclear leukocytes
Abstract
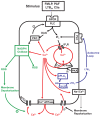
Unlike most other effector cells of the innate, as well as the adaptive immune systems, the neutrophil is a relatively undiscerning aggressor with scant regard for damage limitation. Although this highly combative, professional phagocyte has become increasingly implicated in the immunopathogenesis of many acute and chronic inflammatory disorders, of both infective and noninfective origin, effective pharmacological strategies to counter neutrophil aggression have remained elusive. Activation of neutrophils results in rapid mobilization of both stored and extracellular Ca(2+), resulting in abrupt, usually transient increases in cytosolic Ca(2+), which precede, and are a prerequisite for activation of the Ca(2+)-dependent pro-inflammatory activities of these cells. Mobilization of Ca(2+) by, and restoration of Ca(2+) homeostasis to activated neutrophils are multistep processes which present a number of potential targets, some well recognized and others novel and unconventional, for the pharmacological control of neutrophil-mediated inflammation. Uncovering these targets represents the primary focus of this review.
Keywords: NADPH oxidase; calcium; cyclic AMP; neutrophils; sodium-calcium exchanger.
Figures
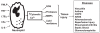
References
-
- Ali H, Sozzani S, Fisher I, et al. Differential regulation of formyl peptide and platelet-activating factor receptors: role of phospholipase Cβ, phosphorylation by protein kinase A. J Biol Chem. 1998;273:11012–16. - PubMed
-
- Anderson R, Steel HC, Tintinger GR. Inositol 1,4,5-triphosphate-mediated shuttling between intracellular stores and the cytosol contributes to the sustained elevation in cytosolic calcium in FMLP-activated human neutrophils. Biochem Pharmacol. 2005;69:1567–75. - PubMed
-
- Arm JP. Leukotriene generation and clinical implications. Allergy Asthma Proc. 2004;25:37–42. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

