Polyethylenimine-mediated gene delivery to the lung and therapeutic applications
- PMID: 19920904
- PMCID: PMC2761186
- DOI: 10.2147/dddt.s2708
Polyethylenimine-mediated gene delivery to the lung and therapeutic applications
Abstract
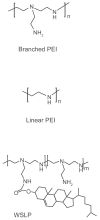
Nonviral gene delivery is now considered a promising alternative to viral vectors. Among nonviral gene delivery agents, polyethylenimine (PEI) has emerged as a potent candidate for gene delivery to the lung. PEI has some advantages over other polycations in that it combines strong DNA compaction capacity with an intrinsic endosomolytic activity. However, intracellular (mainly the nuclear membrane) and extracellular obstacles still hamper its efficiency in vitro and in vivo, depending on the route of administration and the type of PEI. Nuclear delivery has been increased by adding nuclear localization signals. To overcome nonspecific interactions with biological fluids, extracellular matrix components and nontarget cells, strategies have been developed to protect polyplexes from these interactions and to increase target specificity and gene expression. When gene delivery into airway epithelial cells of the conducting airways is necessary, aerosolization of complexes seems to be better suited to guarantee higher transgene expression in the airway epithelial cells with lower toxicity than observed with either intratracheal or intravenous administration. Aerosolization, indeed, is useful to target the alveolar epithelium and pulmonary endothelium. Proof-of-principle that PEI-mediated gene delivery has therapeutic application to some genetic and acquired lung disease is presented, using as genetic material either plasmidic DNA or small-interfering RNA, although optimization of formulation and delivery protocols and limitation of toxicity need further studies.
Keywords: RNA interference; airway epithelial cells; gene therapy; gene transfer; lung; polyethylenimine.
Figures


References
-
- Abdallah B, Hassan A, Benoist C, et al. A powerful nonviral vector for In Vivo gene transfer into the adult mammalian brain: Polyethylenimine. Hum Gene Ther. 1996;7:1947–54. - PubMed
-
- Ahn CH, Chae SY, Bae YH, et al. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J Control Release. 2002;80:273–82. - PubMed
-
- Aigner A, Fischer D, Merdan T, et al. Delivery of unmodified bioactive ribozymes by an RNA-stabilizing polyethylenimine (LMW-PEI) efficiently down-regulates gene expression. Gene Ther. 2002;9:1700–7. - PubMed
-
- Aneja MK, Imker R, Rudolph C. Phage phiC31 integrase-mediated genomic integration and long-term gene expression in the lung after nonviral gene delivery. J Gene Med. 2007;9:967–75. - PubMed
-
- Arote R, Kim TH, Kim YK, et al. A biodegradable poly(ester amine) based on polycaprolactone and polyethylenimine as a gene carrier. Biomaterials. 2007;28:735–44. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

