Ambrisentan for the treatment of pulmonary arterial hypertension
- PMID: 19920913
- PMCID: PMC2761178
- DOI: 10.2147/dddt.s3057
Ambrisentan for the treatment of pulmonary arterial hypertension
Abstract
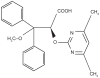
Ambrisentan is an endothelin receptor antagonist (ERA) that was recently approved for treatment of pulmonary arterial hypertension (PAH). Endothelin (ET) is a potent vasoconstrictor with mitogenic, hypertrophic and pro-inflammatory properties that is upregulated in pulmonary hypertensive diseases. The biologic effects of ET are mediated by 2 cell surface receptors termed ET(A) and ET(B). ET(A) mediates the vasoconstrictor effect of ET on vascular smooth muscle, whereas ET(B) is expressed primarily on vascular endothelial cells where it induces nitric oxide synthesis and acts to clear ET from the circulation. Ambrisentan is the first ET(A) selective ERA approved for use in the US. Recently published clinical trials in patients with PAH demonstrate improvement in functional capacity and pulmonary hemodynamics similar to other ET(A) selective and non-selective ERAs. Its once daily dosing and lower incidence of serum aminotransferase elevation offer potential advantages over other ERAs, but further experience with this agent is needed to fully understand its long-term efficacy and safety. This review discusses the endothelin family of proteins and receptors and their role in the pathophysiology of pulmonary hypertensive diseases. It also examines the development process, safety profile and clinical trials that have resulted in ambrisentan being approved for treatment of PAH.
Keywords: ambrisentan; endothelin; endothelin receptor antagonist; pulmonary hypertension.
Figures


Similar articles
-
A review of pulmonary arterial hypertension: role of ambrisentan.Vasc Health Risk Manag. 2007;3(1):11-22. Vasc Health Risk Manag. 2007. PMID: 17583171 Free PMC article. Review.
-
Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension.Expert Opin Pharmacother. 2009 Aug;10(11):1847-58. doi: 10.1517/14656560903061275. Expert Opin Pharmacother. 2009. PMID: 19601701 Review.
-
Oral therapies for the treatment of pulmonary arterial hypertension: a population-based cost-minimization analysis.Appl Health Econ Health Policy. 2009;7(1):43-59. doi: 10.1007/BF03256141. Appl Health Econ Health Policy. 2009. PMID: 19558194
-
Endothelin receptor antagonism in pulmonary arterial hypertension--a role for selective ET(A) inhibition?Curr Med Res Opin. 2006 Dec;22(12):2567-74. doi: 10.1185/030079906X158020. Curr Med Res Opin. 2006. PMID: 17166339 Review.
-
Role of ambrisentan in the management of pulmonary hypertension.Ann Pharmacother. 2008 Nov;42(11):1653-9. doi: 10.1345/aph.1L014. Epub 2008 Oct 28. Ann Pharmacother. 2008. PMID: 18957622 Review.
Cited by
-
An organ-on-chip model of pulmonary arterial hypertension identifies a BMPR2-SOX17-prostacyclin signalling axis.Commun Biol. 2022 Nov 7;5(1):1192. doi: 10.1038/s42003-022-04169-z. Commun Biol. 2022. PMID: 36344664 Free PMC article.
-
Ambrisentan, an endothelin receptor type A-selective antagonist, inhibits cancer cell migration, invasion, and metastasis.Sci Rep. 2020 Sep 28;10(1):15931. doi: 10.1038/s41598-020-72960-1. Sci Rep. 2020. PMID: 32985601 Free PMC article.
-
Endothelin and hepatic wound healing.Pharmacol Res. 2011 Jun;63(6):512-8. doi: 10.1016/j.phrs.2011.03.005. Epub 2011 Mar 21. Pharmacol Res. 2011. PMID: 21421048 Free PMC article. Review.
-
Endothelin-1 promotes cytoplasmic accumulation of RIP140 through a ET(A)-PLCβ-PKCε pathway.Mol Cell Endocrinol. 2012 Apr 4;351(2):176-83. doi: 10.1016/j.mce.2011.12.003. Epub 2011 Dec 19. Mol Cell Endocrinol. 2012. PMID: 22209746 Free PMC article.
-
Insights into ET(A) subtype selectivity of benzodiazepine endothelin receptor antagonists by 3D-QSAR approaches.J Mol Model. 2012 Apr;18(4):1299-311. doi: 10.1007/s00894-011-1153-x. Epub 2011 Jul 12. J Mol Model. 2012. PMID: 21748330
References
-
- Alberts GF, Peifley KA, Johns A, et al. Constitutive endothelin-1 overexpression promotes smooth muscle cell proliferation via an external autocrine loop. J Biol Chem. 1994;269:10112–8. - PubMed
-
- Amberg W, Hergenroder S, Hillen H, et al. LU208075 and LU302146, two novel ETA-selective endothelin receptor antagonists: SAR of 3,3-diaryl propionic acid derivatives. International Conference on Endothelin; 6 October; 1999. pp. 10–13.
-
- Anand I, McMurray J, Cohn J, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–54. - PubMed
-
- Badesch DB, Abman SH, Ahearn GS, et al. Medical therapy for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:35S–62S. - PubMed
-
- Barst RJ, Langleben D, Frost A, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

