Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC
- PMID: 19920917
- PMCID: PMC2769226
Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC
Abstract
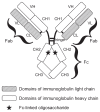
As platforms for therapeutic agents, monoclonal antibodies (MAbs) have already been approved, and several MAbs have demonstrated clinical effectiveness in a variety of malignancies. However, several issues have also been emerging in antibody therapy, such as high cost and insufficient drug action. Recently, to improve MAb activity in humans, effector functions have been subjects of focus, especially antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Extensive efforts have been made to enhance these effector functions of MAbs, and successful approaches have been reported by us and others, wherein the binding activity of MAbs to FcgammaRIIIa or C1q is increased by introducing amino acid mutations into heavy chain constant regions or through glyco-modification of Fc-linked oligosaccharides. In addition, one of the next approaches to optimizing therapeutic antibodies would be to combine multiple enhancing modifications into a single antibody platform to overcome the diverse mechanisms of clinical resistance of tumor cells. For this aim, we have recently developed a successful combination composed of ADCC-enhancing modification by the fucose depletion from Fc-linked oligosaccharides and CDC-enhancing modification by IgG1 and IgG3 isotype shuffling in heavy chains, which could be of great value for the development of third-generation antibody therapeutics.
Keywords: ADCC; CDC; Fc oligosaccharides; IgG isotypes; effector functions; nonfucosylated IgG.
Figures


References
-
- Forero A, Lobuglio AF. History of antibody therapy for non-Hodgkin’s lymphoma. Semin Oncol. 2003;30:1–5. - PubMed
-
- Grillo-López AJ. Rituximab (Rituxan/MabThera): the first decade (1993–2003) Expert Rev Anticancer Ther. 2003;3:767–779. - PubMed
-
- Vogel CL, Franco SX. Clinical experience with trastuzumab (herceptin) Breast J. 2003;9:452–462. - PubMed
-
- de Bono JS, Rowinsky EK. The ErbB receptor family: a therapeutic target for cancer. Trends Mol Med. 2002;8:S19–26. - PubMed
-
- Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

