T10B9 monoclonal antibody: a short-acting nonstimulating monoclonal antibody that spares gammadelta T-cells and treats and prevents cellular rejection
- PMID: 19920935
- PMCID: PMC2769243
- DOI: 10.2147/dddt.s2750
T10B9 monoclonal antibody: a short-acting nonstimulating monoclonal antibody that spares gammadelta T-cells and treats and prevents cellular rejection
Abstract
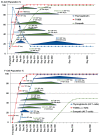
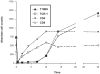
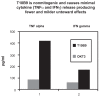
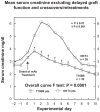
T10B9.1A-31/MEDI-500 is a nonmitogenic immunoglobulin M kappa murine monoclonal antibody (mAb) directed against the alpha-beta (alphabeta) heterodimer of the T-lymphocyte receptor complex. The hybridoma was first produced by fusing spleen cells from BALB/C mice immunized with human peripheral blood T-lymphocytes with SP2/O-Ag14 mutant myeloma cells. The mAb is produced and purified using multistep ion exchange and molecular sieve chromatography protocols. T10B9 has been used successfully to treat acute cellular rejection in renal transplantation and as an immunosuppression induction agent in heart and simultaneous kidney-pancreas transplantation. Because T10B9 is nonmitogenic and causes minimal cytokine release, both treatment of rejection and induction of immunosuppression were accomplished with significantly fewer and milder untoward effects (cytokine release syndrome) than its comparator OKT3. Since T10B9 is directed against the alphabeta heterodimer of the CD3 epitope, it spares the gamma delta (gammadelta) region. These gamma delta (gammadelta) T cells have a unique role in the immune response controlling many serious human diseases and perhaps facilitating the development of immunologic tolerance. T10B9 has a relatively short duration of action, depleting T cells for only 10 to 14 days, unlike the protracted depletion seen with thymoglobulin and Campath-1H. There is no B-lymphocyte depletion with T10B9 as there is with both of the aforementioned reagents. The lack of prolonged lymphocyte depletion may account for less infection observed with T10B9 treatment.
Keywords: Campath-1H; OKT3; T10B9.1A-31; monoclonal antibody; thymoglobulin; γδ T-cell.
Figures

References
-
- Peleg AY, Husain S, Kwak EJ, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007;44(2):204–212. - PubMed
-
- Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26(25):3644–2653. - PubMed
-
- Chakrabarti S, Osman H, Collingham K, Milligan DW. Polyoma viruria following T-cell-depleted allogeneic transplants using Campath-1H: incidence and outcome in relation to graft manipulation, donor type and conditioning. Bone Marrow Transplant. 2003;31(5):379–386. - PubMed
-
- De Santo LS, Romano G, Mastroianni C, et al. Role of immunosuppressive regimen on the incidence and characteristics of cytomegalovirus infection in heart transplantation: a single-center experience with preemptive therapy. Transplant Proc. 2005;37(6):2684–2687. - PubMed
-
- Sepulveda L. Llancaqueo M, Zamorano J, Bermudez C, Cortes C. Cytomegalovirus infections in cardiac transplant patients: 5 and experience at a clinical hospital, university of Chile. Transplant Proc. 2007;39(3):622–624. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

