Safety, efficacy and patient acceptability of the combined estrogen and progestin transdermal contraceptive patch: a review
- PMID: 19920983
- PMCID: PMC2770395
- DOI: 10.2147/ppa.s3233
Safety, efficacy and patient acceptability of the combined estrogen and progestin transdermal contraceptive patch: a review
Abstract
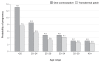
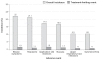
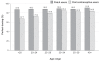
The worldwide introduction of the first, unique patch for hormonal contraception (ethinyl estradiol/norelgestromin, EE/NGMN patch) was widely recognized as a significant event in the development of drug delivery systems. This innovation offers a number of advantages over the oral route, and extensive clinical trials have proved its safety, efficacy, effectiveness, and tolerability. The weekly administration and ease of use/simplicity of the EE/NGMN patch contribute to its acceptability, and help to resolve the two main problems of non-adherence, namely early discontinuation and inconsistent use. The patch offers additional benefits to adolescents (improvement of dysmenorrhea and acne), adults (improvement in emotional and physical well-being, premenstrual syndrome, and menstrual irregularities), and perimenopausal women (correction of hormonal imbalance, modulation of premenopausal symptoms), thus providing high satisfaction rates (in nearly 90% of users). Since its introduction, the transdermal contraceptive patch has proved to be a useful choice for women who seek a convenient formulation which is easy to use, with additional, non-contraceptive tailored benefits for all the ages.
Keywords: hormonal contraceptive; patient adherence; patient satisfaction; transdermal.
Figures
References
-
- Abrams LS, Skee DM, Natarajan J, et al. Pharmacokinetics of norelgestromin and ethinyl estradiol delivered by a contraceptive patch (Ortho Evra™/Evra™) under conditions of heat, humidity and exercise. J Clin Pharmacol. 2001;41:1301–9. - PubMed
-
- Archer DF, Bigrigg A, Smallwood GH, et al. Assessment of compliance with a weekly contraceptive patch (Ortho Evra™/Evra™) among North American women. Fertil Steril. 2002;77(2, Suppl 2):S27–S30. - PubMed
-
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra®) on contraceptive efficacy. Contraception. 2004;69:189–95. - PubMed
-
- Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. A randomized controlled trial. JAMA. 2001;285:2347–54. - PubMed
-
- Burkman RT. Transdermal hormonal contraception: benefits and risks. Am J Obstet Gynecol. 2007;197:134e1–6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

