Exacerbation of facial motoneuron loss after facial nerve axotomy in CCR3-deficient mice
- PMID: 19922414
- PMCID: PMC2826103
- DOI: 10.1042/AN20090017
Exacerbation of facial motoneuron loss after facial nerve axotomy in CCR3-deficient mice
Abstract
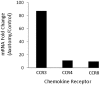
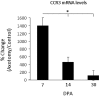
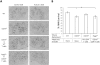
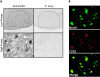
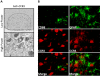
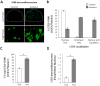
We have previously demonstrated a neuroprotective mechanism of FMN (facial motoneuron) survival after facial nerve axotomy that is dependent on CD4(+) Th2 cell interaction with peripheral antigen-presenting cells, as well as CNS (central nervous system)-resident microglia. PACAP (pituitary adenylate cyclase-activating polypeptide) is expressed by injured FMN and increases Th2-associated chemokine expression in cultured murine microglia. Collectively, these results suggest a model involving CD4(+) Th2 cell migration to the facial motor nucleus after injury via microglial expression of Th2-associated chemokines. However, to respond to Th2-associated chemokines, Th2 cells must express the appropriate Th2-associated chemokine receptors. In the present study, we tested the hypothesis that Th2-associated chemokine receptors increase in the facial motor nucleus after facial nerve axotomy at timepoints consistent with significant T-cell infiltration. Microarray analysis of Th2-associated chemokine receptors was followed up with real-time PCR for CCR3, which indicated that facial nerve injury increases CCR3 mRNA levels in mouse facial motor nucleus. Unexpectedly, quantitative- and co-immunofluorescence revealed increased CCR3 expression localizing to FMN in the facial motor nucleus after facial nerve axotomy. Compared with WT (wild-type), a significant decrease in FMN survival 4 weeks after axotomy was observed in CCR3(-/-) mice. Additionally, compared with WT, a significant decrease in FMN survival 4 weeks after axotomy was observed in Rag2(-/-) (recombination activating gene-2-deficient) mice adoptively transferred CD4(+) T-cells isolated from CCR3(-/-) mice, but not in CCR3(-/-) mice adoptively transferred CD4(+) T-cells derived from WT mice. These results provide a basis for further investigation into the co-operation between CD4(+) T-cell- and CCR3-mediated neuroprotection after FMN injury.
Figures
References
-
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. - PubMed
-
- Baird JW, Nibbs RJ, Komai-Koma M, Connolly JA, Ottersbach K, Clark-Lewis I, Liew FY, Graham GJ. ESkine, a novel β-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J Biol Chem. 1999;274:33496–33503. - PubMed
-
- Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. - PubMed
-
- Byram SC, Serpe CJ, Pruett SB, Sanders VM, Jones KJ. Natural killer cells do not mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:417–425. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
