Influence of GSTP1 I105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma
- PMID: 19922504
- PMCID: PMC11158438
- DOI: 10.1111/j.1349-7006.2009.01418.x
Influence of GSTP1 I105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma
Abstract
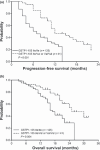
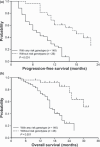
Glutathione S-transferase P1 (GSTP1) participates in detoxification of potentially genotoxic compounds that may alter the efficacy and toxicity of platinum-based chemotherapy. We analyzed the influence of I105V polymorphism of GSTP1 on clinico-pathological features and outcomes in 166 Chinese patients with metastatic colorectal carcinoma who had been treated with first-line FOLFOX-4. Combined analysis of GSTP1 I105V, ERCC1-118, and XPD-751 polymorphisms was also conducted. The results showed that, in comparison with Caucasian populations, a remarkably lower prevalence of Val105 allele variants was noted (24.7%). Patients with Val105 allele variants had a higher response to FOLFOX-4 (56.1%vs 37.6%, P = 0.04), and a longer progression-free (P < 0.01) as well as overall (P < 0.01) survival. By adjusted analysis, this polymorphism was identified as an independent prognostic factor (P = 0.01). In combined analysis, patients without any risk genotype, including GSTP1-105 Ile/Ile, ERCC1-118 C/T or T/T, and XPD-751 Lys/Gln, had significantly longer progression-free and overall survivals (P < 0.01). In addition, patients with Val105 allele variants had a higher incidence of grade 3/4 cumulative neuropathy after different cycles of treatment. These data suggest that Asian populations have a lower prevalence of I105V polymorphism in GSTP1. I105V polymorphism in GSTP1, by reducing its enzymatic activity and consequential detoxification to oxaliplatin, could be a key determinant for a better outcome, but more neurotoxicity, to FOLFOX-4 treatment.
Figures
References
-
- Rixe O, Ortuzar W, Alvarez M et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug‐resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem Pharmacol 1996; 52: 1855–65. - PubMed
-
- De Gramont A, Figer A, Seymour M et al. Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47. - PubMed
-
- Andre T, Boni C, Mounedji‐Boudiaf L et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. - PubMed
-
- Cassidy J, Tabernero J, Twelves C et al. XELOX (capecitabine plus oxaliplatin): active first‐line therapy for patients with metastatic colorectal cancer. J Clin Oncol 2004; 22: 2084–91. - PubMed
-
- Tournigand C, Cervantes A, Figer A et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop‐and‐go fashion in advanced colorectal cancer – a GERCOR study. J Clin Oncol 2006; 24: 394–400. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

