Impaired immune responses in the lungs of aged mice following influenza infection
- PMID: 19922665
- PMCID: PMC2785782
- DOI: 10.1186/1465-9921-10-112
Impaired immune responses in the lungs of aged mice following influenza infection
Abstract
Background: Each year, influenza virus infection causes severe morbidity and mortality, particularly in the most susceptible groups including children, the elderly (>65 years-old) and people with chronic respiratory diseases. Among the several factors that contribute to the increased susceptibility in elderly populations are the higher prevalence of chronic diseases (e.g. diabetes) and the senescence of the immune system.
Methods: In this study, aged and adult mice were infected with sublethal doses of influenza virus (A/Puerto Rico/8/1934). Differences in weight loss, morbidity, virus titer and the kinetics of lung infiltration with cells of the innate and adaptive immune responses were analyzed. Additionally, the main cytokines and chemokines produced by these cells were also assayed.
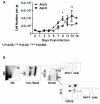
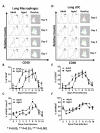
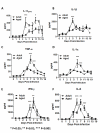
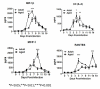
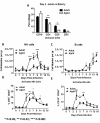
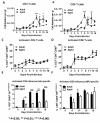
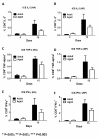
Results: Compared to adult mice, aged mice had higher morbidity, lost weight more rapidly, and recovered more slowly from infection. There was a delay in the accumulation of granulocytic cells and conventional dendritic cells (cDCs), but not macrophages in the lungs of aged mice compared to adult animals. The delayed infiltration kinetics of APCs in aged animals correlated with alteration in their activation (CD40 expression), which also correlated with a delayed detection of cytokines and chemokines in lung homogenates. This was associated with retarded lung infiltration by natural killer (NK), CD4+ and CD8+ T-cells. Furthermore, the percentage of activated (CD69+) influenza-specific and IL-2 producer CD8+ T-cells was higher in adult mice compared to aged ones. Additionally, activation (CD69+) of adult B-cells was earlier and correlated with a quicker development of neutralizing antibodies in adult animals.
Conclusion: Overall, alterations in APC priming and activation lead to delayed production of cytokines and chemokines in the lungs that ultimately affected the infiltration of immune cells following influenza infection. This resulted in delayed activation of the adaptive immune response and subsequent delay in clearance of virus and prolonged illness in aged animals. Since the elderly are the fastest growing segment of the population in developed countries, a better understanding of the changes that occur in the immune system during the aging process is a priority for the development of new vaccines and adjuvants to improve the immune responses in this population.
Figures



References
-
- Bender BS, Taylor SF, Zander DS, Cottey R. Pulmonary immune response of young and aged mice after influenza challenge. J Lab Clin Med. 1995;126(2):169–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

