Epitopes for broad and potent neutralizing antibody responses during chronic infection with human immunodeficiency virus type 1
- PMID: 19922969
- PMCID: PMC2789835
- DOI: 10.1016/j.virol.2009.10.044
Epitopes for broad and potent neutralizing antibody responses during chronic infection with human immunodeficiency virus type 1
Abstract
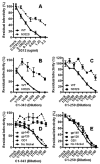
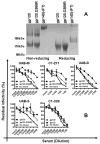
Neutralizing antibody (nAb) response is sporadic and has limited potency and breadth during infection with human immunodeficiency virus type 1 (HIV-1). In rare cases, broad and potent nAbs are actually induced in vivo. Identifying specific epitopes targeted by such broad and potent nAb response is valuable in guiding the design of a prophylactic vaccine aimed to induce nAb. In this study, we have defined neutralizing epitope usage in 7 out of 17 subjects with broad and potent nAbs by using targeted mutagenesis in known neutralizing epitopes of HIV-1 glycoproteins and by using in vitro depletion of serum neutralizing activity by various recombinant HIV-1 glycoproteins. Consistent with recent reports, the CD4 binding site (CD4BS) is targeted by nAbs in vivo (4 of the 7 subjects with defined neutralizing epitopes). The new finding from this study is that epitopes in the gp120 outer domain are also targeted by nAbs in vivo (5 of the 7 subjects). The outer domain epitopes include glycan-dependent epitopes (2 subjects), conserved nonlinear epitope in the V3 region (2 subjects), and a CD4BS epitope composed mainly of the elements in the outer domain (1 subject). Importantly, we found indication for epitope poly-specificity, a dual usage of the V3 and CD4BS epitopes, in only one subject. This study provides a more complete profile of epitope usage for broad and potent nAb responses during HIV-1 infection.
Figures


References
-
- Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82 (23):11651–68. - PMC - PubMed
-
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–7. - PubMed
-
- Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433(7028):834–41. - PubMed
-
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206 (2):935–44. - PubMed
-
- Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med. 2005;201 (9):1407–19. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

