Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation
- PMID: 19922971
- PMCID: PMC2789909
- DOI: 10.1016/j.virol.2009.10.040
Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation
Abstract
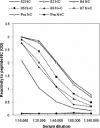
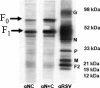
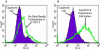
Human respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract infection in infants, immunocompromised patients, and the elderly. The RSV fusion (F) protein mediates fusion of the viral envelope with the target cell membrane during virus entry and is a primary target for antiviral drug and vaccine development. The F protein contains two heptad repeat regions, HR1 and HR2. Peptides corresponding to these regions form a six-helix bundle structure that is thought to play a critical role in membrane fusion. However, characterization of six-helix bundle formation in native RSV F protein has been hindered by the fact that a trigger for F protein conformational change has yet to be identified. Here we demonstrate that RSV F protein on the surface of infected cells undergoes a conformational change following exposure to elevated temperature, resulting in the formation of the six-helix bundle structure. We first generated and characterized six-helix bundle-specific antibodies raised against recombinant peptides modeling the RSV F protein six-helix bundle structure. We then used these antibodies as probes to monitor RSV F protein six-helix bundle formation in response to a diverse array of potential triggers of conformational changes. We found that exposure of 'membrane-anchored' RSV F protein to elevated temperature (45-55 degrees C) was sufficient to trigger six-helix bundle formation. Antibody binding to the six-helix bundle conformation was detected by both flow cytometry and cell-surface immunoprecipitation of the RSV F protein. None of the other treatments, including interaction with a number of potential receptors, resulted in significant binding by six-helix bundle-specific antibodies. We conclude that native, untriggered RSV F protein exists in a metastable state that can be converted in vitro to the more stable, fusogenic six-helix bundle conformation by an increase in thermal energy. These findings help to better define the mechanism of RSV F-mediated membrane fusion and have important implications for the identification of therapeutic strategies and vaccines targeting RSV F protein conformational changes.
Figures















References
-
- Anderson K, Stott EJ, Wertz GW. Intracellular processing of the human respiratory syncytial virus fusion glycoprotein: amino acid substitutions affecting folding, transport and cleavage. J Gen Virol. 1992;73(Pt 5):1177–88. - PubMed
-
- Baker KA, Dutch RE, Lamb RA, Jaredtzky TS. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell. 1999;3(3):309–319. - PubMed
-
- Barretto N, Hallak LK, Peeples ME. Neuraminidase treatment of respiratory syncytial virus-infected cells or virions, but not target cells, enhances cell-cell fusion and infection. Virology. 2003 Aug 15;313(1):33–43. 2003. - PubMed
-
- Begona Ruiz-Arguello M, Gonzalez-Reyes L, Calder LJ, Palomo C, Martin D, Saiz MJ, Garcia-Barreno B, Skehel JJ, Melero JA. Effect of proteolytic processing at two distinct sites on shape and aggregation of an anchorless fusion protein of human respiratory syncytial virus and fate of the intervenig segment. Virology. 2002 Jul 5;298(2):317–26. 2002. - PubMed
-
- Behera AK, Matsuse H, Kumar M, Kong X, Lockey RF, Mohapatra SS. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem. Biophys. Res. Commun. 2001;280(1):188–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

