Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables
- PMID: 19923174
- PMCID: PMC2812355
- DOI: 10.1128/JVI.01482-09
Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables
Abstract
Induction of antibodies that neutralize a broad range of human immunodeficiency virus type 1 (HIV-1) isolates is a major goal of vaccine development. To study natural examples of broad neutralization, we analyzed sera from 103 HIV-1-infected subjects. Among progressor patients, 20% of sera neutralized more than 75% of a panel of 20 diverse viral isolates. Little activity was observed in sera from long-term nonprogressors (elite controllers). Breadth of neutralization was correlated with viral load, but not with CD4 count, history of past antiretroviral use, age, gender, race/ethnicity, or route of exposure. Clustering analysis of sera by a novel method identified a statistically robust subgrouping of sera that demonstrated broad and potent neutralization activity.
Figures

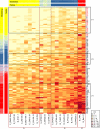
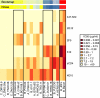
References
-
- Bailey, J. R., K. G. Lassen, H. C. Yang, T. C. Quinn, S. C. Ray, J. N. Blankson, and R. F. Siliciano. 2006. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 80:4758-4770. - PMC - PubMed
-
- Beirnaert, E., P. Nyambi, B. Willems, L. Heyndrickx, R. Colebunders, W. Janssens, and G. van Der Groen. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 62:14-24. - PubMed
-
- Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651-11668. - PMC - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

