Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis
- PMID: 19923187
- PMCID: PMC2812336
- DOI: 10.1128/JVI.02029-09
Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis
Abstract
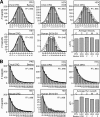
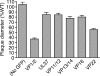
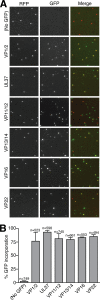
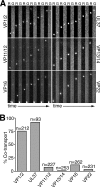
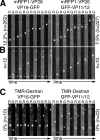
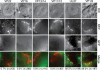
Upon entry, neuroinvasive herpesviruses traffic from axon terminals to the nuclei of neurons resident in peripheral ganglia, where the viral DNA is deposited. A detailed analysis of herpes simplex virus type 1 (HSV-1) transport dynamics in axons following entry is currently lacking. Here, time lapse fluorescence microscopy was used to compare the postentry viral transport of two neurotropic herpesviruses: HSV-1 and pseudorabies virus (PRV). HSV-1 capsid transport dynamics were indistinguishable from those of PRV and did not differ in neurons of human, mouse, or avian origin. Simultaneous tracking of capsids and tegument proteins demonstrated that the composition of actively transporting HSV-1 is remarkably similar to that of PRV. This quantitative assessment of HSV-1 axon transport following entry demonstrates that HSV-1 and PRV share a conserved mechanism for postentry retrograde transport in axons and provides the foundation for further studies of the retrograde transport process.
Figures
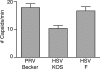

References
-
- Blyth, W. A., D. A. Harbour, and T. J. Hill. 1984. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J. Gen. Virol. 65:1477-1486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

