Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation
- PMID: 19923190
- PMCID: PMC2812326
- DOI: 10.1128/JVI.01326-09
Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation
Abstract
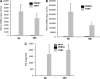
Porcine reproductive and respiratory syndrome virus (PRRSV) infection of swine leads to a serious disease characterized by a delayed and defective adaptive immune response. It is hypothesized that a suboptimal innate immune response is responsible for the disease pathogenesis. In the study presented here we tested this hypothesis and identified several nonstructural proteins (NSPs) with innate immune evasion properties encoded by the PRRS viral genome. Four of the total ten PRRSV NSPs tested were found to have strong to moderate inhibitory effects on beta interferon (IFN-beta) promoter activation. The strongest inhibitory effect was exhibited by NSP1 followed by, NSP2, NSP11, and NSP4. We focused on NSP1alpha and NSP1beta (self-cleavage products of NSP1 during virus infection) and NSP11, three NSPs with strong inhibitory activity. All of three proteins, when expressed stably in cell lines, strongly inhibited double-stranded RNA (dsRNA) signaling pathways. NSP1beta was found to inhibit both IFN regulatory factor 3 (IRF3)- and NF-kappaB-dependent gene induction by dsRNA and Sendai virus. Mechanistically, the dsRNA-induced phosphorylation and nuclear translocation of IRF3 were strongly inhibited by NSP1beta. Moreover, when tested in a porcine myelomonocytic cell line, NSP1beta inhibited Sendai virus-mediated activation of porcine IFN-beta promoter activity. We propose that this NSP1beta-mediated subversion of the host innate immune response plays an important role in PRRSV pathogenesis.
Figures

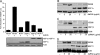
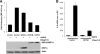
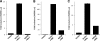

References
-
- Albina, E., C. Carrat, and B. Charley. 1998. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 18:485-490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

